This article was co-authored by wikiHow staff writer, Hunter Rising. Hunter Rising is a wikiHow Staff Writer based in Los Angeles. He has more than three years of experience writing for and working with wikiHow. Hunter holds a BFA in Entertainment Design from the University of Wisconsin - Stout and a Minor in English Writing.
There are 14 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 1,843 times.
Learn more...
Most of the elements on the periodic table are solids, but could you pick out which ones are different from the rest? While the only 2 elements that are liquids at standard room temperature are mercury (Hg) and bromine (Br), there are a few others that melt when it’s slightly warmer. There are even more elements that exist as gasses instead. If you want to find out more about all of the liquid or gaseous elements and their properties, just keep reading.
Things You Should Know
- Mercury and bromine are the 2 elements that are liquid at room temperature, which is around 77 °F (25 °C).
- Francium, cesium, gallium, and rubidium melt into liquids just above room temperature.
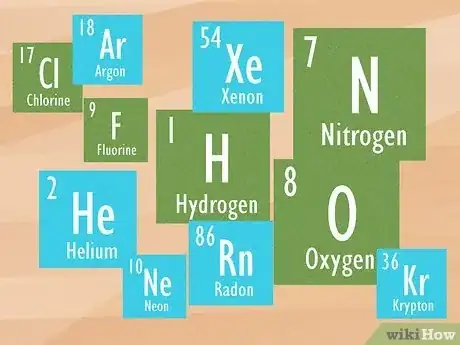
- 11 elements are gaseous at room temp, including hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon, and radon.
Steps
Liquids at Room Temperature
-
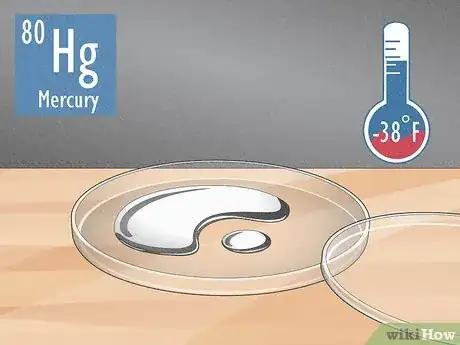
1Mercury (Hg, atomic number 80) This metallic silver metal has a melting point of just −38 °F (−39 °C), so it’ll be a liquid at standard room temperature, which is around 77 °F (25 °C).[1] Mercury is naturally-occurring, and it was once used inside thermometers, dental fillings, and batteries. However, inhaling the vapors mercury produces can cause lung damage and kidney problems, so it’s toxic if you’re exposed to high levels.[2]
-
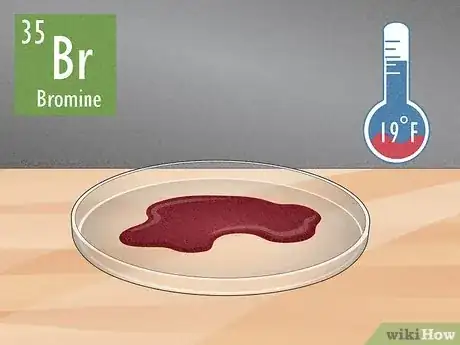
2Bromine (Br, atomic number 35) Bromine melts into a deep-red, oily liquid with a bleach-like odor when it reaches 19 °F (−7 °C).[5] Bromine is usually used in flame retardants and agricultural pesticides, but exposure to the pure element or the fumes it produces is toxic.[6]
- Exposure to bromine could cause eye and throat irritation, and cause painful sores on your skin.
Liquids Near Room Temperature
-
1Francium (Fr, atomic number 87) While francium is a solid metal at room temperature, it melts into a liquid when it reaches 81 °F (27 °C). Francium is an alkali metal, so it’s highly reactive and doesn’t exist in its pure form often on Earth. Since it’s a rarer element, scientists primarily study francium and don't use it outside of a lab setting.[7]
-
2Cesium (Cs, atomic number 55) Cesium is a silvery white metal, and it melts into a liquid at 83.5 °F (28.6 °C).[8] Cesium has the largest atoms of any element and the highest density compared to other alkali metals. When it’s exposed to air, cesium ignites into a reddish-violet flame.[9]
- Pure cesium also reacts violently with water.
-
3Gallium (Ga, atomic number 31) Gallium is a soft gray metal that melts when it reaches 86.3 °F (30.2 °C). Just holding gallium in your hand will warm it up enough to turn it into a liquid. Gallium isn’t toxic, but handling it still may cause some skin irritation.[10]
-
4Rubidium (Rb, atomic number 37) Rubidium is a silvery-white metallic element that melts when it reaches 102.7 °F (39.3 °C). Rubidium is normally used in lab settings since it can spontaneously ignite when it’s exposed to air or water.[11]
Warnings
- Many of the elements that are liquids are toxic or radioactive if you’re exposed to them. Avoid handling them unless you’re in a safe lab setting.[15]⧼thumbs_response⧽
References
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Mercury#section=Melting-Point
- ↑ https://www.epa.gov/mercury/basic-information-about-mercury
- ↑ https://www.betterhealth.vic.gov.au/health/healthyliving/mercury-exposure-and-poisoning#where-to-get-help
- ↑ https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- ↑ https://emergency.cdc.gov/agent/bromine/basics/facts.asp
- ↑ https://www.rsc.org/periodic-table/element/35/bromine
- ↑ https://pubchem.ncbi.nlm.nih.gov/element/Francium#section=Element-Period-Number
- ↑ https://pubsapp.acs.org/cen/80th/cesium.html?
- ↑ https://www.atsdr.cdc.gov/ToxProfiles/tp157-c4.pdf
- ↑ https://antoine.frostburg.edu/chem/senese/101/periodic/faq/liquid-elements.shtml
- ↑ https://periodic.lanl.gov/37.shtml
- ↑ https://engineering.mit.edu/engage/ask-an-engineer/why-is-mercury-liquid-at-room-temperature/
- ↑ https://chem.libretexts.org/Courses/Howard_University/General_Chemistry%3A_An_Atoms_First_Approach/Unit_4%3A__Thermochemistry/Chapter_10%3A_Gases/Chapter_10.1%3A_Gaseous_Elements_and_Compounds
- ↑ https://www.researchgate.net/figure/Gibbs-free-energies-of-the-solid-green-liquid-blue-and-gas-phases-orange-of-Cn_fig2_336389017
- ↑ https://antoine.frostburg.edu/chem/senese/101/periodic/faq/liquid-elements.shtml






-Electric-Shock-Step-9.webp)