This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
This article has been viewed 128,046 times.
A hydrometer is a simple device that allows you to measure the density of various liquids relative to water. Hydrometers are used for a myriad of different purposes: they are used to measure the fat content of milk, the alcohol content of beer, wine, and spirits, and even the water content of urine to test for dehydration. To make a hydrometer, you need a tube, some wax, and some paper.
Steps
Making the Hydrometer
-
1Mark your intervals. Use a pencil to mark down the edge of a piece of paper. You should have a mark every 2 millimetres (0.079 in). You will need to use a ruler to accurately measure out 2 millimetres (0.079 in).
- Make enough marks to be roughly the length of your index finger.
-
2Cut a strip from the paper. Cut the side of the paper that you marked on into a long, thin strip. This will allow you to slide the paper into a piece of glass tubing. The tubing should be open on both ends.Advertisement
-
3Insert the strip of paper into a glass tube. Use a tube roughly the size of a drinking straw. If you are doing this at home and do not have a glass tube, you can substitute the glass tube with a clear straw. A colored straw would make it difficult to read the markings on your paper.
-
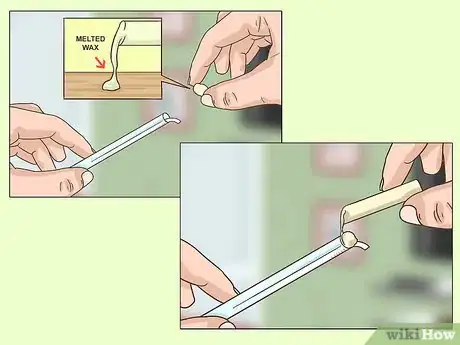
4Seal one end of the tubing. Melt wax or glue to secure the paper on one side of the tube. Then, use the glue or wax to make an airtight seal on that side of the tube. Leave the other end open.
-
5Cut any excess paper. The paper strip should be flush with the bottom of the tube. Use scissors to cut the strip. Be careful not to pull or tug on the strip or you might break it.
Calibrating the Hydrometer
-
1Add water to a graduated cylinder. It does not matter how much water you add to the cylinder. Roughly 100 milliliters (3 fl oz) will do fine. Make sure the water is as pure and free of contaminants as possible.[1]
- Any clear bottle, such as a pop bottle, will work as well.
-
2Add weight. Place 4-5 grams of metal in the tube. This will reduce the buoyancy of the tube enough for part of the tube to sink. This is necessary to use the instrument for measurements.
- One source of metal that is readily available is a small nail.
-
3Insert the glass tube or straw into the water. The sealed side should go into the water first. Avoid getting water in the tubing. This will change the buoyancy of the tube and get your paper wet.[2]
- You can change how much the tube sinks or floats by removing or adding weight (metal).
-
4Mark the water level. Use a permanent marker to make a line on the side of your glass tube at exactly the level of the water. This will serve as a point of reference for the instrument and be designated as 1.0. This mark shows how far the tube sinks in a liquid that has the exact density of water.[3]
Determining the Relative Density of Other Liquids
-
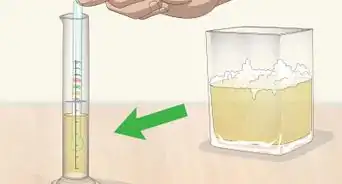
1Submerge the hydrometer in liquid. Choose a liquid to test. Put roughly 100 milliliters (3 fl oz) into a beaker. Place the hydrometer into the beaker.[4]
-
2Mark the level of the liquid. Just as you marked the surface of the water level, make a mark to show how high the liquid in question comes on the hydrometer. You can tell a little about the relative density just by this initial mark. For example, if this mark is above the 1.0 mark, the liquid is less dense than water and if it is below the 1.0 mark it is denser than water.
- Less dense liquids will allow the tube to sink farther and more dense liquids will allow the tube to sink less, due to the Archimedes Principle, which states that the buoyant force of an object submerged in water is equal to the weight of the water that was displaced.[5]
-
3Derive the relative density. If you want a more specific relative density, you can derive it mathematically. Measure the distance from the bottom of the tube to the 1.0 mark and the distance to the new mark. Divide the distance of the 1.0 mark by the distance of the new mark. This will give you the relative density of the liquid to water.
- For example, if your 1.0 mark is 5 millimetres (0.20 in) from the bottom and your new mark is 4.5 millimetres (0.18 in) from the bottom, you would divide 5 by 4.5 (or 0.20 by 0.18, if using inches). This gives you a relative density of roughly 1.1. Your liquid is 1.1 times as dense as water.
- In the other direction, if your 1.0 mark is 5 millimetres (0.20 in) from the bottom and your new mark is 5.5 millimetres (0.22 in) from the bottom, you would divide 5 by 5.5 (or 0.20 by 0.22, if using inches). This gives you a relative density of roughly 0.9. Your liquid is 0.9 times as dense as water.
Community Q&A
-
QuestionHow can this determine the alcohol content in homemade wine?
 MidnightBlue1_1Top AnswererProperly measuring the alcohol content of homemade wine or beer requires the use of a device called a hydrometer.
MidnightBlue1_1Top AnswererProperly measuring the alcohol content of homemade wine or beer requires the use of a device called a hydrometer. -
QuestionMaybe it is just a matter of convention, but liquids with density less than water will have a number smaller than 1, and liquids with a density greater than water would have a number greater than 1?
 HannahCommunity AnswerIt depends on what you mean. If the density of the liquid is greater than that of water, the hydrometer will sink less - resulting in a number below the 1,0 mark on the hydrometer and vice versa. If you measure density of that substance in e.g. kilograms per liter, the number will be above 1 (which is the density of water).
HannahCommunity AnswerIt depends on what you mean. If the density of the liquid is greater than that of water, the hydrometer will sink less - resulting in a number below the 1,0 mark on the hydrometer and vice versa. If you measure density of that substance in e.g. kilograms per liter, the number will be above 1 (which is the density of water). -
QuestionMy hydrometer always sinks to the bottom of the graduated cylinder. Is that supposed to happen?
 RayCommunity AnswerThe hydrometer has a definite mass and volume. By the principle of Archimedes, it floats when it displaces an equal mass of liquid. Since the lighter liquid has less mass per volume, a larger volume of liquid must be displaced. For the hygrometer to displace a larger volume, it sinks further.
RayCommunity AnswerThe hydrometer has a definite mass and volume. By the principle of Archimedes, it floats when it displaces an equal mass of liquid. Since the lighter liquid has less mass per volume, a larger volume of liquid must be displaced. For the hygrometer to displace a larger volume, it sinks further.
Warnings
- Some liquids are highly flammable, toxic, or corrosive. Exercise caution when working with any dangerous or unknown liquids.⧼thumbs_response⧽
References
- ↑ https://cdn.dal.ca/content/dam/dalhousie/pdf/faculty/science/imhotep/7.9%20Making%20things%20Float%20and%20Making%20a%20Hydrometer.pdf
- ↑ https://ceramicartsnetwork.org/daily/article/How-to-Make-a-Floating-Stick-Hydrometer-for-the-Cost-of-a-Milkshake
- ↑ https://www.exploratorium.edu/snacks/eyedropper-hydrometer
- ↑ https://www.exploratorium.edu/snacks/eyedropper-hydrometer
- ↑ https://www.livescience.com/58839-archimedes-principle.html






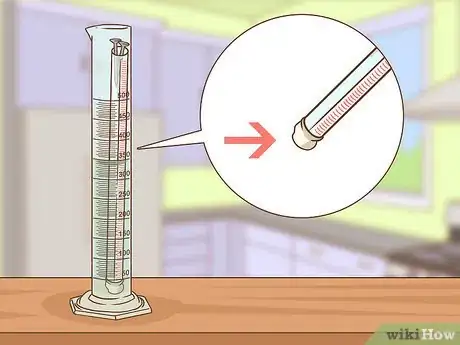






-Step-15.webp)