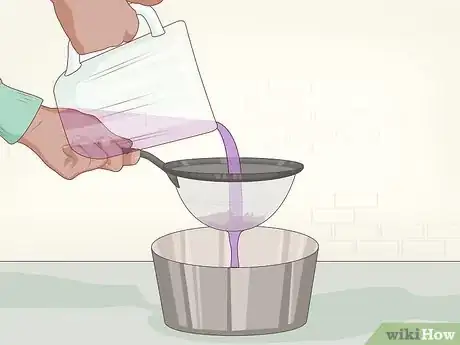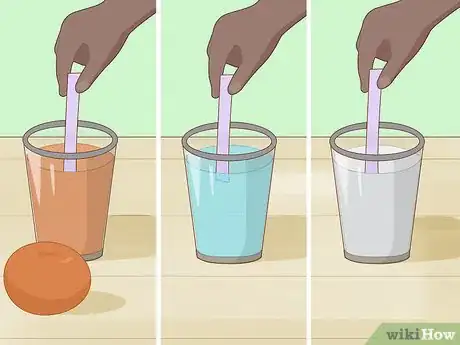This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 13 testimonials and 85% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 497,238 times.
The pH scale measures how likely a substance is to give up protons (or H+ ions) and how likely that substance is to accept protons. Many molecules, including dyes, will change their structure by either accepting protons from an acidic environment (one that readily gives up protons) or donating protons to a basic environment (one that readily accepts protons). Testing for pH is an essential part of many chemistry and biology experiments. This test can be done by coating paper strips with dyes that will change into different colors in the presence of an acid or a base.
Steps
Making Homemade pH Paper with Cabbage
-
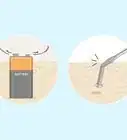
1Chop up some red cabbage. You'll need to chop about ¼ of a head of red cabbage and put it in a blender. You will extract chemicals from the cabbage to coat your pH paper. These chemicals are known as anthocyanins and are found in plants such as cabbage, roses, and berries. Anthocyanins are purple under neutral conditions (pH 7.0) but they change color when exposed to an acid (pH < 7.0) or a base (pH>7.0).[1]
- The same procedure can be followed using berries, roses, and other anthocyanin containing plants.
- This does not work for green cabbage. The same anthocyanins are not present in green cabbage.
-
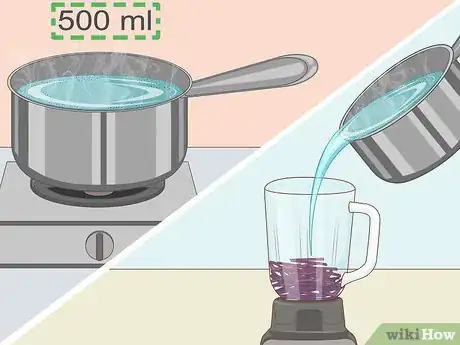
2Add boiling water to your cabbage. You can boil the water on a stovetop or in the microwave, but either way you'll need about 500 mL of water. Pour the boiling water directly into the blender with the cabbage. This will help draw the needed chemicals out of the cabbage.[2]Advertisement
-
3Turn on the blender. You need to blend the water and cabbage for best results. Keep the mixture blending until the water is dark purple. This color change indicates that you have successfully drawn the needed chemicals (anthocyanins) from the cabbage and dissolved them in the hot water. You should allow the contents of the blender to cool for at least ten minutes before proceeding.[3]
-
4Pour the mix through a strainer. You want to remove any pieces of cabbage from the indicator solution (colored water). Filter paper will work in place of a strainer, but may take more time. Once you have strained the indicator solution, you can throw away the cabbage pieces.[4]
-
5Add isopropyl alcohol to your indicator solution. Adding about 50 mL of isopropyl alcohol will protect your solution from bacterial growth. The alcohol may start to alter the color of your solution. If this happens, add vinegar until the solution goes back to dark purple.[5]
- You can substitute ethanol for isopropyl alcohol, if necessary or desired.
-
6Pour the solution into a pan or bowl. You want a container with a wide enough opening to dip your paper. You should choose a container that is stain-resistant, as you are pouring dyes into it. Ceramic and glass are good options.[6]
-
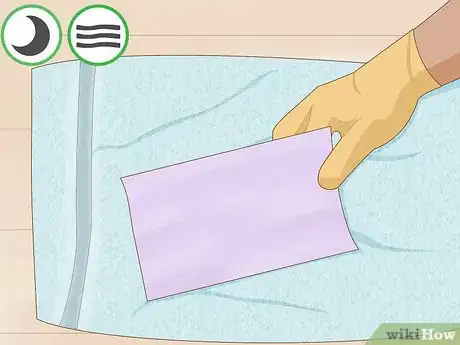
7Soak your paper in the indicator solution. Make sure to push the paper all the way to the bottom. You want to cover all corners and edges of the paper. It is a good idea to use gloves for this step.[7]
-
8Allow your paper to air dry on a towel. Find a location that is free of acidic or basic vapors. The paper should be allowed to dry completely before proceeding. Ideally, you will leave it overnight.[8]
-
9Cut the paper into strips. This will allow you to test several different samples. You can cut the strips any size you would like, but generally the length and width of your index finger is fine. This will allow you to dip the strip into a sample without getting your fingers into the sample.[9]
-
10Use the strips to test the pH of different solutions. You can test household solutions such as orange juice, water, and milk. You can also mix up solutions for testing, e.g. mixing water and baking soda. This will give you a wide range of samples to test.[10]
-
11Store the strips in cool dry place. You should use an airtight container to store the strips until you use them. This will protect them from environmental contamination such as acidic or basic gases. It is also ideal not to leave them in direct sunlight, as this could result in bleaching over time.[11]
Making Homemade Litmus Paper
-
1Obtain dry litmus powder. Litmus is a compound that is derived from lichens, fungi that form symbiotic relationships with alga and/or cyanobacteria capable of photosynthesis. You can purchase litmus powder online or at a chemical supply store.
- It is possible to make your own litmus powder if you are a competent chemist. However, the process is quite involved and includes adding several compounds such as lime and potash to ground lichens and allowing weeks for fermentation.
-
2Dissolve the litmus into water. Make sure to stir the solution and heat if the powder is not dissolving well. The litmus powder needs to dissolve completely into the water. The resulting solution should be a violet-blue color.
-
3Submerge white acid-free art paper in the litmus solution. Get all sides and corners of the paper wet with the solution. This will give you the most surface area on the test strip and provide the most accurate results. You do not need to leave the paper to “soak” as long as you ensure that it is coated thoroughly.
-
4Allow your paper to dry. You should dry the paper in the open air, but be sure that you are not exposing it to acidic or basic vapors. These vapors could contaminate the strips and make the inaccurate. You should also be sure to store them in a dry, dark place to prevent contamination and bleaching.[12]
-
5Use the litmus paper to test for acidity. Blue litmus papers turn red in the presence of an acid. Keep in mind that they will not indicate how strong the acid is, or whether a solution is basic. No change means that the solution is either basic or neutral, but not acidic.[13]
- You can make red litmus paper (that turns blue when exposed to a base) by adding acid to the indicator solution before soaking your paper.
Expert Q&A
Did you know you can get expert answers for this article?
Unlock expert answers by supporting wikiHow
-
QuestionWhere's a good place to buy litmus paper cheaply?
 Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Environmental Scientist
-
QuestionWhat kind paper should I use?
 Community AnswerUse acid-free paper -- most paper products have this labeled on them if they are acid-free. Check in the arts and crafts or the paper and printing sections of your local stationery stores or supermarkets.
Community AnswerUse acid-free paper -- most paper products have this labeled on them if they are acid-free. Check in the arts and crafts or the paper and printing sections of your local stationery stores or supermarkets. -
QuestionIs hydrogen peroxide a base or an acid?
 Community AnswerHydrogen peroxide is neither a base nor an acid because it does not fully or partially dissociate in water to form hydrogen ions or hydroxide ions.
Community AnswerHydrogen peroxide is neither a base nor an acid because it does not fully or partially dissociate in water to form hydrogen ions or hydroxide ions.
Warnings
- Keep the prepared strips in a cool, dark, dry airtight container.⧼thumbs_response⧽
- Handle the test strips with clean dry hands only.⧼thumbs_response⧽
- Handle any acids with great care and under supervision of a responsible person, such as a science teacher if you are doing a class project. Wear appropriate gear for handling any of the substances.⧼thumbs_response⧽
Things You'll Need
- Distilled water
- Earthenware dish or plate
- Chosen vegetable or mineral matter
- Paper strips
- Clean, dry drying area
- It works best with red cabbage
- Isopropyl Alcohol
References
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p041/chemistry/make-your-own-ph-paper
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p041/chemistry/make-your-own-ph-paper
- ↑ https://www.acs.org/content/dam/acsorg/pressroom/podcasts/ph-test-strips-activity.pdf
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p041/chemistry/make-your-own-ph-paper
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p041/chemistry/make-your-own-ph-paper
About This Article
To make homemade pH paper test strips, start by chopping up 1/4 head of red cabbage. If you want, you could substitute red berries or red roses. Place the chopped cabbage in a blender and add in some boiling water. Blend this together and then pour it through a strainer to remove any solids. After you've strained the solution, place the liquid in a bowl and add in some isopropyl alcohol to prevent bacterial growth. Then, soak your paper in the solution and let the paper air dry before you cut it into pH strips. To learn how to make homemade litmus paper, read on!