This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 94% of readers who voted found the article helpful, earning it our reader-approved status.
This article has been viewed 202,423 times.
Electroplating is a process used to coat one metal with that of a separate metal. There are many objects in our everyday lives that have been plated with other metals: gold-plated jewelry, nickel and copper plated coins, etc. The process involves passing an electric current through a solution of electrolytes that allows for the transfer of metal ions from the donor metal to the recipient metal.[1] You can easily setup a simple apparatus at home to electroplate your own metals.
Steps
Electroplating with Muriatic Acid
-
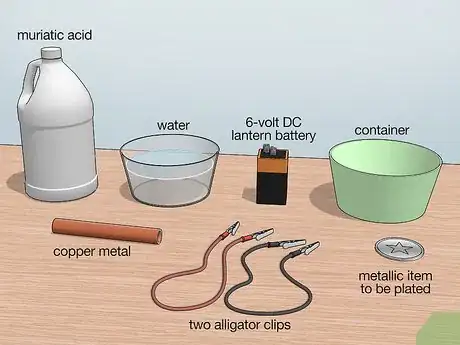
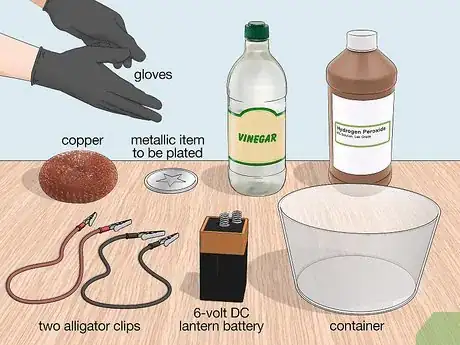
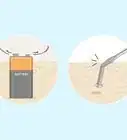
1Gather the necessary materials. To setup this system, you will need water, muriatic acid, a 6-volt DC lantern battery, two alligator clips, a piece of copper metal, the metallic item you are trying to plate, and a container large enough for the metal you want to electroplate to be submerged. All of these items can be found at a local hardware or home improvement store.
- The 6-volt battery has two prongs that make it easier to connect to the system, but you can use a battery of lower voltage.
- Alligator clips are insulated electrical wires that have metal clips as the ends used for connecting circuits together.
- The piece of copper is the source of ions that will be used to plate your metal item.
- Steel and nickel are two metals that can easily be plated with copper.
-
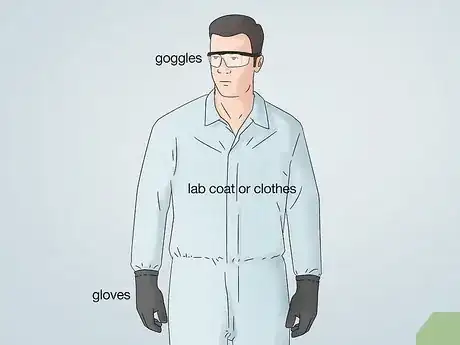
2Wear proper protective equipment. When electroplating metals, you are dealing with acids and other chemicals that you need to be protected from. Wear protective eye goggles, gloves, and a lab coat or clothes that you don’t mind ruining if something splashes.Advertisement
-
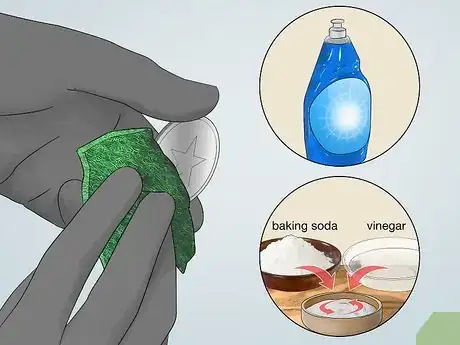
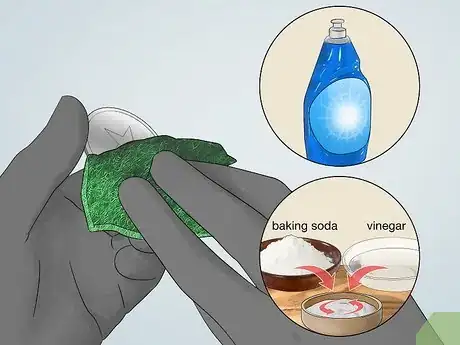
3Scrub the metal surface clean. To get an even electroplated coat, you must first clean the surface of your metal object. Dirt and oils on the surface can prevent the donor metal from coating the surface. Start with a degreaser such as a detergent (dish soap) and then scrub the metal with an abrasive, acidic cleaner to ensure the surface is very clean.[2]
- A mixture of vinegar and baking soda can be used as an acidic cleaner to scrub the surface clean.
-
4Measure 5 parts water. To make the electroplating solution you will mix 5 parts water with 1 part muriatic acid. Never add water directly to acid! Adding water to acid causes an exothermic reaction that can lead to the acid exploding and be very harmful. You want to fill a container large enough to submerge both pieces of metal that will be used for the electroplating process. Start with 5 cups of water and add more as necessary.[3]
- Always maintain the ratio of 5:1. For example, if you need more than 5 cups, measure out 10 cups of water and add 2 cups of muriatic acid.
-
5Add 1 part muriatic acid to the water. To avoid injury, remember to always add the acid to the water. If you’ve measured out 5 cups of water, measure out 1 cup of acid and slowly add it to the water while stirring.[4]
- Remember to keep the ratio of 5:1 water to acid.
- The outside of the container will be warm because adding acid to water is exothermic (it creates heat).
- Stir with a glass or plastic stirrer because the acid will degrade a metal.
-
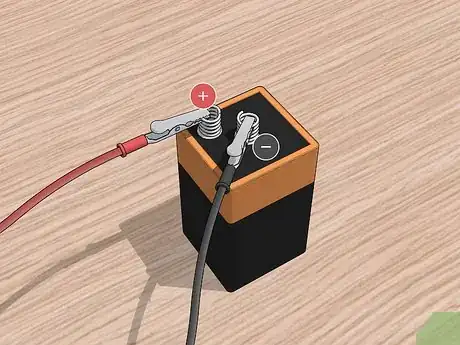
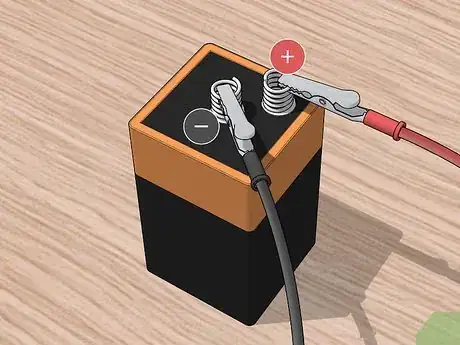
6Connect one alligator clip to each prong of the battery supply. The battery will serve to produce the current necessary for the electroplating process. Attach one clip to one prong and the second clip to the second prong of the battery.
- At this stage, it doesn’t matter which clip is connected to which terminal, just that a separate clip is attached to each one.
-
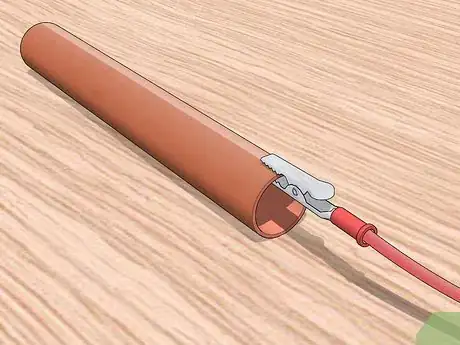
7Connect the copper to the positive terminal of the battery. Using an alligator clip, attach the positive terminal of the battery to the piece of copper. If you clip the copper piece to the wrong terminal, the electroplating will not work.[5]
- On a standard 6-volt battery, the positive terminal is the male side of the battery.
-
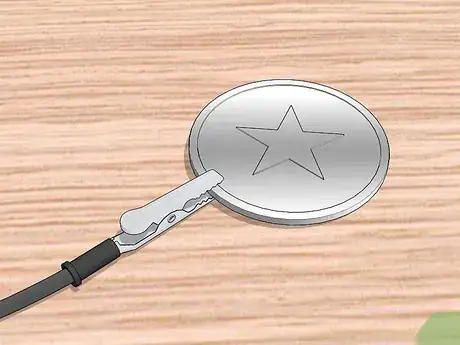
8Connect the metal piece to be plated to the negative terminal of the battery. Take the clip attached to the negative terminal of the battery and attach it to the metal you are trying to plate. Clip the pieces in the least conspicuous way possible. If there is no out-of-the-way place to attach the clip, you will need to alter its location during the plating process so the piece of metal gets evenly coated.[6]
- The negative side of a standard 6-volt battery is the female component.
- If you attach the positive clip to the metal, the electroplating will not work. Double check that you have the correct terminals attached.
-
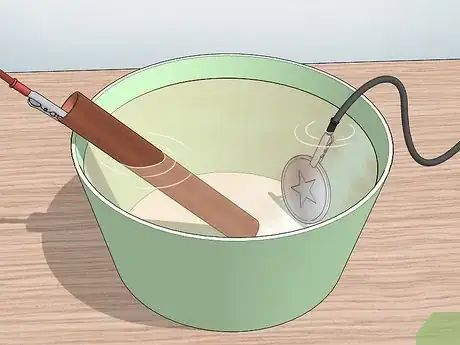
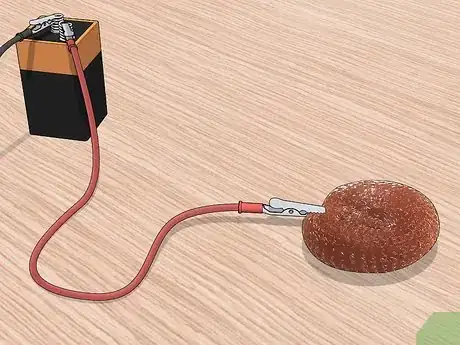
9Submerge both metals into the diluted muriatic acid bath. Once everything is connected, you can place both metal pieces into the bath. The copper piece doesn’t have to be fully submerged, but the piece you are plating does.
- Stir the solution during the plating process to get an even coat.
- Keep the two metal pieces at least an inch apart from each other to avoid getting burn spots where the copper accumulates too quickly.
-
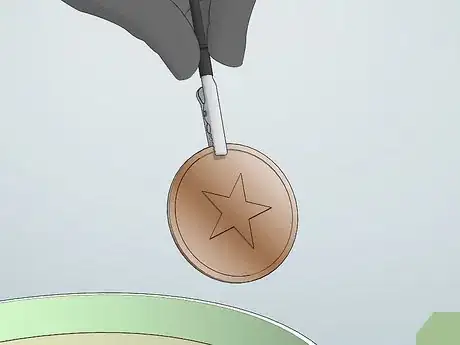
10Remove metal piece when you are satisfied with the coat. Using this method makes it a little more difficult to get a thick coat of copper, but you will be able to get a thin, even coat. When are you are satisfied with the look of the metal you were trying to plate, you can remove the metal from the bath and place it on a towel to dry.
- The plating can take anywhere from a few minutes to a few hours.
- If you have more metals to plate, you can connect the next metal to the clip, place it in the acid bath, and stir to coat.
Electroplating with a Metal Ion Electrolyte Solution
-
1Gather the necessary materials. To electroplate with this method, you will need a piece of copper, the metal to be plated, vinegar, hydrogen peroxide, alligator clips, a 6-volt lantern battery, a glass/plastic container, and gloves. All of these items can be purchased at a local hardware or home improvement store.
- A cheap and easy way to obtain 100% copper is to use a copper scrubbing sponge that can be found in the cleaning aisle of the supermarket.
- Alligator clips are electrical wires that have metal clips on each end used for connecting circuits.
- You can use a battery with a voltage as low as 1.5 volts if you don’t have a lantern battery.
- Use a container large enough to submerge the metal that you’re trying to plate.
-
2Combine and heat equal parts vinegar and hydrogen peroxide. You will need enough of this solution to submerge your to-be-plated metal. To make four cups of solution, add two cups of vinegar to two cups of hydrogen peroxide.[7]
- Combining vinegar and hydrogen peroxide makes peracetic acid which must be handled with caution.[8]
-
3Soak half of the copper scrubbing pad in the vinegar-peroxide solution. The solution will turn blue, indicating that the scrub has dissolved into the solution. You now have a copper ion solution that can be used for electroplating.[9]
- Wear gloves from this step on because the copper solution is toxic.
- Soak the copper until the solution turns a light blue. It is better for the solution to be of weak concentration, so remove the copper scrub before the solution gets too dark.
-
4Attach one end of each alligator clip to the terminals of the battery. The battery provides the current necessary to transport the metals from the donor to the recipient. Connect one alligator clip to the positive terminal of the battery and another alligator clip to the negative terminal.
- At this stage, it doesn’t matter which clip is connected to which terminal, as long as there is a separate clip attached to each one.
-
5Clean the metal to be electroplated. Before starting the electroplating method, you must ensure that the metal is clean so the new metal atoms can form a solid bond to the recipient metal.[10] Wear gloves to both protect your hands and keep the metal surface clean. It is essential to do this step well for an even electroplate.
- Clean the grease with a mild detergent such as dish soap.
- Scrub the metal surface with an acidic, abrasive cleaner. A 50-50 vinegar and baking soda solution works well to clean copper.[11]
- Rinse the surface well to remove any residue and pat dry with a paper towel.
- From this point on, handle the metal only with gloves on to avoid contaminating the surface.
-
6Connect the positive alligator clip to the copper scrub. Take the other half of the copper scrub that wasn’t used to make the solution. Attach the alligator clip connected to the positive terminal of the battery and connect it to the copper scrub. If you attach it to the negative terminal, the electroplating won’t work.[12]
-
7Connect the negative alligator clip to the to-be-plated metal. The other clip should be connected to the negative terminal of the battery. Attach this clip to the metal that you are plating. Try to attach it in a place that is inconspicuous.
- You may need to move the clip during the plating process, so you get an even coat.
- If you attach the metal to be plated to the positive terminal, the electroplating won’t work. Simply switch the clips and the process will work.
-
8Submerge the metals in the blue copper solution. Once both metals have been connected, submerge them in the blue copper solution you already made. Because they are connected to the battery, a current is flowing through the circuit. Atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat.[13]
- To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and keep the solution in constant motion.
-
9Remove the metal when the coat is even. When you are satisfied with the look of the plating on your metal, remove it from the solution. You can take it out of the solution and check the coat, then replace it in the solution without disrupting the process.
- Rinse the metal and set on a towel to dry.
Community Q&A
-
QuestionCan I use a copper electroplate on semiprecious gemstones and glass to make jewelry?
 Paul SCommunity AnswerYou cannot directly electroplate the gemstones themselves, but the jewelry they are mounted on can be plated. It is best to plate the jewelry without the gemstones, then mount the stones in the plated material.
Paul SCommunity AnswerYou cannot directly electroplate the gemstones themselves, but the jewelry they are mounted on can be plated. It is best to plate the jewelry without the gemstones, then mount the stones in the plated material. -
QuestionCan I use nickels for a donor metal to nickel plate items?
 Community AnswerNo, nickels are not pure nickel, they are a alloy. I think Amazon sells pure nickel strips.
Community AnswerNo, nickels are not pure nickel, they are a alloy. I think Amazon sells pure nickel strips. -
QuestionIs electroplating with muriatic acid easier than plating with a metal ion electrolyte?
 Paul SCommunity AnswerNo, since extreme caution must be used, along with personal protection such as gloves, goggles, and apron. Good ventilation of the area you are working in is also very important. The used acid will also be hazardous.
Paul SCommunity AnswerNo, since extreme caution must be used, along with personal protection such as gloves, goggles, and apron. Good ventilation of the area you are working in is also very important. The used acid will also be hazardous.
Warnings
- Wash your hands after handling electrolyte solution, which may cause irritation to eyes and skin if not rinsed off immediately.⧼thumbs_response⧽
References
- ↑ http://www.explainthatstuff.com/electroplating.html
- ↑ https://technotes.alconox.com/industry/medical-device/acid-vs-alkaline-cleaner/
- ↑ https://www.youtube.com/watch?v=SIU3lsZMx7A
- ↑ https://www.youtube.com/watch?v=SIU3lsZMx7A
- ↑ http://www.explainthatstuff.com/electroplating.html
- ↑ https://www.youtube.com/watch?v=SIU3lsZMx7A
- ↑ http://hackaday.com/2013/11/01/copper-electroplating-the-cheap-and-safe-way/
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S0022391320307538
- ↑ http://hackaday.com/2013/11/01/copper-electroplating-the-cheap-and-safe-way/
About This Article
Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. You’ll need muriatic acid, a 6-volt DC lantern battery, 2 alligator clips, a piece of copper metal, and a container large enough to submerge the metal you want to electroplate. First, you’ll need to scrub the surface of your metal with dish soap and an abrasive cleaner to remove any dirt and oil. Then, dilute your muriatic acid with water in your container. Connect your battery's positive terminal to the copper and the negative one to your metal, and submerge both metals in the acid solution. When you’re satisfied with the look of its coating, remove it and leave it to dry. This can take anywhere from a few minutes to a few hours. Don’t forget to wear goggles and gloves to protect yourself from the acid. For more tips from our Science co-author, including how to electroplate metal with a metal ion electrolyte solution, read on!