This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
This article has been viewed 198,198 times.
Aluminum is a readily available metal that is used in a wide range of products. Aluminum alloys (pure aluminum mixed with other metals) are used in everything from cooking utensils to household furnishings and vehicle parts. The surface layer of an aluminum piece also forms a strong bond with oxygen from the air. This protects the aluminum and makes it more durable, but can also cause a discolored or dull appearance. Acid can often be used to neutralize the oxidation on the aluminum surface to restore the bright, shiny appearance of aluminum.
Steps
Acid Washing Aluminum
-
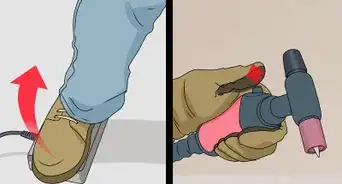
1Apply the acid wash to the surface of your aluminum. This step will depend on the size of your piece and the stain you are trying to remove. If you have a stain that covers a large portion of the aluminum, it is often best to soak the piece in the acid for 1 to 2 hours. If you are removing a small stain or do not have a tank big enough for your piece to fit in, you can put your acid on a rag and rub gently back and forth.[1]
- Do not go in a circular motion, as this can cause the aluminum to look uneven in the finished product.
-
2Scrub lightly with a soft abrasive if needed. If the stain does not come off easily with just acid, consider using salt or baking soda as a minimal abrasive. You can rub it in with a rag. Put as little force into the scrubbing as possible to minimize scratches on your aluminum surface. [2]
- Sometimes, steel wool is used as a more serious abrasive. If you feel that you need to do this, you should look for the finest grade of steel wool that you can buy and be very gentle with it. Scratches in your aluminum will allow things to get stuck on even worse in the future.
Advertisement -
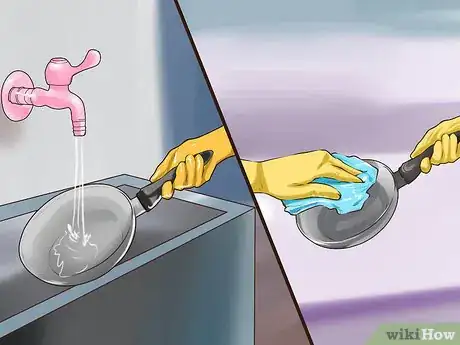
3Rinse the acid away and dry the piece. If you leave acid on your piece it can eventually damage it and cause pitting. Rinse the piece in room temperature (about 70 °F (21 °C)) water. Once the acid is removed, simply dry the piece with a soft, clean towel.[3]
-
4Protect the aluminum from future damage by polishing it. You can find an aluminum polish at your local home improvement store, or order one online. Apply the polish by rubbing it in a circular motion with a rag, and then removing it with another rag. Buff the surface with a clean rag to shine up your piece.[4]
- Do not put aluminum polish on any surface that will come in contact with food or fire. It is flammable and toxic.
Making an Acid Cleaning Solution
-
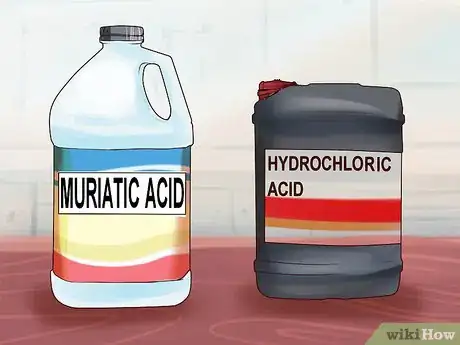
1Choose an appropriate acid. Muriatic acid, otherwise known as hydrochloric acid, is a common choice for an acid wash. It is reasonably safe for the aluminum and fairly easy to come by. Keep in mind that this acid is very dangerous and should be kept away from pets and children. It is also toxic to the environment.[5]
- Muriatic acid isn’t pure hydrochloric acid and doesn’t have a standard concentration. Make sure to always check the product label to know the exact concentration.[6]
- Wear gloves and protective eyewear to avoid any irritation.
- Another approach is to make an acid solution from vinegar or cream of tartar and water. This is safer than using muriatic acid or other strong acids.[7]
-
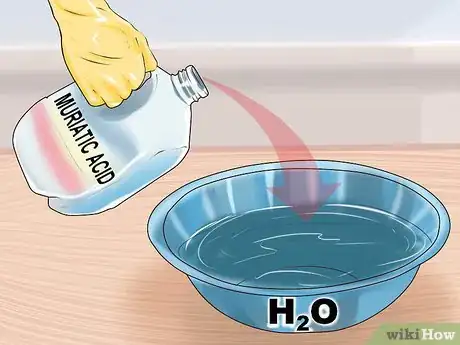
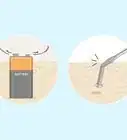
2Pour your acid into water to dilute it. It is very important that you do this correctly. When water and acid mix, a large amount of heat is generated. As long as you pour the acid into a container of water, the heat will be safely dispersed.[8] Consult the label or manufacturer for dilution ratios of water to acid.
- If you pour the water into the acid, the initial mixture is very concentrated acid, and it may get hot enough to flash boil, sending concentrated acid spewing out of the container. Pouring acid into water prevents this and protects you from flash boiling.[9]
-
3Keep the acid wash at room temperature. Room temperature is ideal for the acid to remove grime and corrosion from your aluminum. This works out great if you have to scrub the piece, as dealing with very hot or very cold acid could be difficult. You should also be sure that the aluminum piece is at room temperature before attempting to clean it with acid.
- You can also boil a dilute acid solution (e.g. 1 tablespoon (15 ml) of vinegar in 1 US quart (950 ml) of water) in a corroded pot or pan and then rinse and wipe it clean.[10]
Pre-Washing Aluminum
-
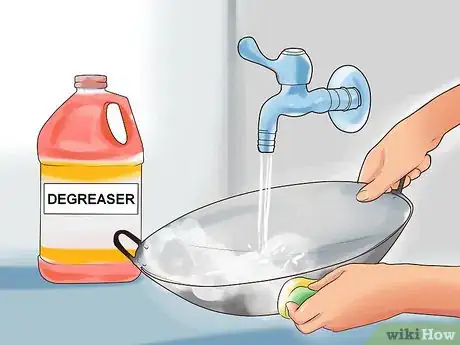
1Use warm water and a degreaser to wash the aluminum surface. The goal is to remove as much dirt and grime as possible. If you are trying to clean corrosion off of your aluminum piece, the acid will need to be able to reach the corrosion to do its job. Washing as many contaminants as possible from the piece prepares it for the acid wash.[11]
-
2Choose a light abrasive for any needed scrubbing. A little bit of scrubbing might be needed to remove things such as burnt-on food. If this is the case, you want to use the softest method that will work. Baking soda rubbed with a rag is a good idea. Remember to use a back and forth motion rather than a circular motion to ensure an even look.[12]
-
3Rinse and dry the piece thoroughly before the acid wash. Once you have washed and scrubbed the piece, it is ready for the acid wash. Rinse off any residues left behind by things such as detergent or baking soda. Dry the aluminum surface with a soft rag before exposing it to the acid solution.[13]
Expert Q&A
Did you know you can get expert answers for this article?
Unlock expert answers by supporting wikiHow
-
QuestionWhat happens when aluminum reacts with hydrochloric acid?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
-
QuestionHow do you remove oxidation from aluminum?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
-
QuestionWhat can I use to clean aluminum?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
Warnings
- It is best to wear gloves when working with acids even if they are diluted. If you are working with a strong acid, such as hydrochloric acid, you should wear gloves, goggles, and other skin protection.⧼thumbs_response⧽
- If using a strong acid such as hydrochloric acid, the fumes can be dangerous. Wear a respirator or work under a ventilated hood.⧼thumbs_response⧽
- Avoid harsh chemicals when possible.[15]⧼thumbs_response⧽
Things You’ll Need
- Acid
- Protective eyewear
- Gloves
- Water
- Rags
- Abrasives
- A Piece of Aluminum
- Detergent
References
- ↑ http://www.howtocleanstuff.net/how-to-clean-aluminum/
- ↑ http://www.howtocleanthings.com/surfaces/how-to-clean-aluminum/
- ↑ http://www.howtocleanstuff.net/how-to-clean-and-polish-aluminum/
- ↑ http://www.howtocleanstuff.net/how-to-clean-and-polish-aluminum/
- ↑ http://www.howtocleanstuff.net/how-to-clean-aluminum/
- ↑ https://www.thoughtco.com/what-is-muriatic-acid-608510
- ↑ http://www.howtocleanstuff.net/how-to-clean-and-polish-aluminum/
- ↑ http://antoine.frostburg.edu/chem/senese/101/safety/faq/always-add-acid.shtml
- ↑ http://antoine.frostburg.edu/chem/senese/101/safety/faq/always-add-acid.shtml
- ↑ http://www.howtocleanstuff.net/how-to-clean-aluminum/
- ↑ http://www.howtocleanstuff.net/how-to-clean-aluminum/
- ↑ http://www.howtocleanthings.com/surfaces/how-to-clean-aluminum/
- ↑ http://www.howtocleanthings.com/surfaces/how-to-clean-aluminum/
- ↑ http://www.howtocleanstuff.net/how-to-clean-and-polish-aluminum/
- ↑ http://www.howtocleanthings.com/surfaces/how-to-clean-aluminum/
About This Article
To acid wash aluminum if the aluminum has oxidation stains, start by putting on safety gear such as gloves and protective eyewear. Next, make an acid cleaning solution by pouring hydrochloric acid into a container of water. Be sure to follow the instructions on the label so you know how much water and acid to use! Then, soak the aluminum in the acid cleaning solution for 1 to 2 hours. If it’s a stubborn stain, remove the aluminum from the acid and rub salt or baking soda into the stain using a rag. Once the aluminum is clean, rinse away the acid with room temperature water and dry it with a clean towel. For more information from our Biochemistry co-author, like how to pre-wash aluminum, read on!