wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
This article has been viewed 72,844 times.
Learn more...
Galvanizing steel coats it with a layer of zinc to protect it from corrosion. Zinc was first used in construction around the time of the destruction of Pompeii, but it was first used to galvanize steel (actually iron) in 1742 and the process patented in 1837.[1] Galvanized steel is used in making sheet metal flashing, gutters, and downspouts, as well as for exterior nails. There are several processes that can be used to galvanize steel: hot-dip galvanizing, electrogalvanizing, sherardizing, and metallic spraying.[2]
Steps
Hot-Dip Galvanizing
-
1Clean away the surface contaminants. Before any other steps can be taken, the steel surface must be cleaned thoroughly. How this is done depends on what has to be cleaned away.
- Dirt, grease, oil, or paint markings require the use of a mild acid, a hot alkali, or a biological cleaning agent.
- Asphalt, epoxy, vinyl, or the slag from welding need to be cleaned by sandblasting or with other abrasives.
-
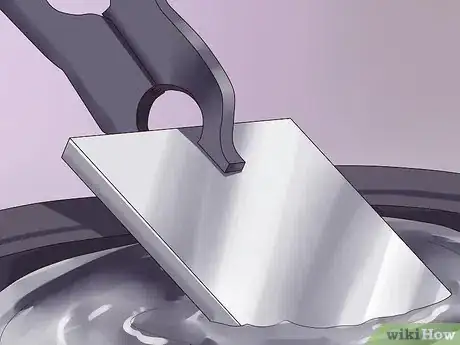
2Pickle away the rust. Pickling is done with hydrochloric acid or hot sulfuric acid; it removes both rust and mill scale.
- In some cases, abrasive cleaning may be enough to remove the rust, or it may be necessary to use both a pickling solution and abrasives. In some cases, larger abrasives such as buckshot are air-blasted onto the steel.
Advertisement -
3Put the metal in flux. In this case, “flux” is a solution of zinc ammonium chloride that removes any remaining rust and scale and protects the steel from rusting until it is actually galvanized.
-
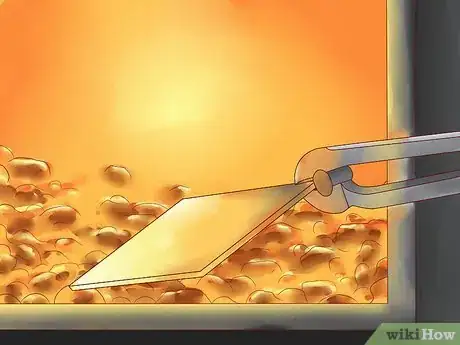
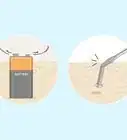
4Immerse the steel in molten zinc. The bath of molten zinc should be at least 98 percent zinc and maintained at a temperature range of 815 to 850 degrees F (435 to 455 degrees C).
- While the steel is immersed in the zinc bath, its iron reacts with the zinc to form a series of alloy layers and an outer layer of pure zinc.
-
5Take the galvanized steel out of the zinc bath slowly. Most of the excess zinc will drain off; what doesn’t drain off can be vibrated off or spun off in a centrifuge.
-
6Cool the galvanized steel. Cooling the metal stops the galvanization reaction, which continues as long as the steel is the same temperature it was while immersed in the zinc bath. Cooling can be done in one of several ways:[3]
- Immerse the steel in a passivation solution such as potassium hydroxide.[4]
- Immerse the steel in water.
- Let the steel cool in the open air.
-
7Inspect the galvanized steel. Once the galvanized steel is cooled, check it to make sure the zinc coating looks good, sticks to the steel, and is thick enough. There are a number of tests that can be performed to ensure the galvanization was successful.
- Standards for hot-dip galvanizing and inspecting its results have been established by such organizations as the American Society for Testing and Materials (now called ASTM International),[5] the International Standards Organization (ISO), the Canadian Standards Association (CSA), and the American Association of State Highway and Transportation Officials (AASHTO). :[6]
Electrogalvanizing
-
1Prepare the steel as for hot-dip galvanizing. The steel must be cleaned and de-rusted before the electrogalvanization can occur.
-
2Prepare a zinc electrolyte solution. Either zinc sulfate or zinc cyanide is normally used for the electrolyte.
-
3Immerse the steel in the electrolyte. The solution will react with the steel to cause the zinc to precipitate onto the steel, coating it. The longer the steel is left in the electrolyte, the thicker the coating that will be produced.
- While this method offers greater control over how thick the zinc coating is than does hot-dip galvanizing, it usually does not allow for layers to become as thick.[7]
Sherardizing
-
1Prepare the steel as with the other galvanization methods. Clean away the dirt with acid or sandblasting as necessary and pickle away the rust.
-
2Place the steel in an airless enclosure.
-
3Surround the steel with powdered zinc.
-
4Heat the steel. This melts the powdered zinc into a liquid that, when cooled, leaving a thin alloy coating.
- Sherardizing is best used for shaped steel pieces, as the galvanic coating will follow the configurations of the steel underneath. It is best used with fairly small metal objects.[8]
Metallic Spraying
-
1Prepare the steel as with the other methods. Clean off all the dirt and remove the rust so it’s ready to be sprayed.
-
2Spray on a fine molten zinc coating.
-
3Heat the coated steel to ensure proper bonding.
- Galvanic coatings produced with this method are less brittle and less prone to peeling and flaking, but provide less protection from rusting for the steel underneath.[9]
Community Q&A
-
QuestionWhat is the purpose of coating steel with zinc?
 GabrielCommunity AnswerGalvanizing, or zinc coating, steel and iron metals protects the metal from corrosion and prevents rust. It is very similar to electroplating, anodizing, tinning and powder-coating metallic surfaces from exposure to the elements. Contact with air and saltwater also corrodes or oxidizes. Oxygen reacts with metal and the surface turns the metal layer into metallic oxides (rust), which is prevented with the zinc coating.
GabrielCommunity AnswerGalvanizing, or zinc coating, steel and iron metals protects the metal from corrosion and prevents rust. It is very similar to electroplating, anodizing, tinning and powder-coating metallic surfaces from exposure to the elements. Contact with air and saltwater also corrodes or oxidizes. Oxygen reacts with metal and the surface turns the metal layer into metallic oxides (rust), which is prevented with the zinc coating. -
QuestionHow long does steel stay molten when galvanizing?
 Community AnswerThe zinc is molten at a temperature of approximately 850° F; steel doesn't melt until much higher (2500°+ F), so the steel shouldn't be molten. Even in the case of iron its melting point is approximately 2200° F, so that shouldn't be an issue.
Community AnswerThe zinc is molten at a temperature of approximately 850° F; steel doesn't melt until much higher (2500°+ F), so the steel shouldn't be molten. Even in the case of iron its melting point is approximately 2200° F, so that shouldn't be an issue. -
QuestionCan I apply galvanized coating on steel that has a primer coat?
 Community AnswerAny impurities on the surface of the steel prior to galvanizing would prevent the reaction you're wanting; or, at the very least, would provide a poor result. It should be as clean as possible before galvanizing, as any impurities on the surface could have a negative effect.
Community AnswerAny impurities on the surface of the steel prior to galvanizing would prevent the reaction you're wanting; or, at the very least, would provide a poor result. It should be as clean as possible before galvanizing, as any impurities on the surface could have a negative effect.
Warnings
- The zinc coating from galvanized steel is vulnerable to corrosion from acids and alkalines (bases). It is particularly vulnerable to sulfuric and sulfurous acids, which can be produced by the mixing of hydrogen sulfide and sulfur dioxide with rainwater (acid rain), made worse if the rain has run off wooden shingles or moss. Rainwater may also react with the zinc coating to form zinc carbonate. Over time, the zinc carbonate will become brittle and eventually expose zinc alloy or even the base metal underneath to corrosion.⧼thumbs_response⧽
- Galvanized steel is harder to paint than non-galvanized steel.⧼thumbs_response⧽
- Galvanized steel has little resistance to corrosion by being in contact with any metal other than aluminum, lead, tin, or zinc. It is especially prone to corrosion around iron, steel, and copper, as well as next to cements containing chlorides or sulfates.⧼thumbs_response⧽
- The zinc coating in galvanized steel is also vulnerable to metal fatigue, because zinc is prone to expand when heated and contract when cooled. [12]⧼thumbs_response⧽
References
- ↑ http://www.galvanizeit.org/hot-dip-galvanizing/what-is-hot-dip-galvanizing-hdg
- ↑ http://www.gsa.gov/portal/content/111758
- ↑ http://www.galvanizeit.org/hot-dip-galvanizing/what-is-hot-dip-galvanizing-hdg
- ↑ http://www.sciencedirect.com/science/article/pii/0013468695800085
- ↑ http://www.astm.org/ABOUT/overview.html
- ↑ http://www.galvanizeit.org/hot-dip-galvanizing/what-is-hot-dip-galvanizing-hdg
- ↑ http://www.gsa.gov/portal/content/111758
- ↑ http://www.gsa.gov/portal/content/111758
- ↑ http://www.gsa.gov/portal/content/111758