Chapter 22
Transition Metals
By Boundless

Iron, the active site of many redox enzymes, has many oxidation states, but ferrous (Fe2+) and ferric (Fe3+) are the most common.

Copper is a ductile metal that conducts heat and electricity and forms a rich variety of compounds with oxidation states +1 and +2.

Chromium exhibits a wide range of possible oxidation states, where the +3 state is the most stable energetically.
The most common oxidation states of the metal manganese are +2, +3, +4, +6, and +7; the +2 oxidation state is the most stable.

Silver has the highest electrical conductivity of any element and the highest thermal conductivity of any metal.

Mercury is a heavy, silvery d-block metal that forms weak bonds and is a liquid at room temperature.
Transition metals typically form several oxidation states and therefore have several oxidation numbers.
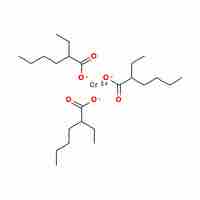
Transition-metal and coordination compounds are named using a set of rules that describe oxidation numbers and anion and cation composition.

Coordination stereoisomers have the same bonds in different orientations; structural isomers have different bonding orientations.

The coordination number determines the number of ligands attached to the central ion and the overall shape of the complex.
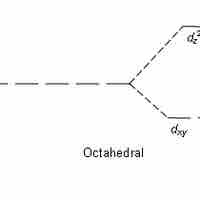
Crystal field theory states that d or f orbital degeneracy can be broken by the electric field produced by ligands, stabilizing the complex.
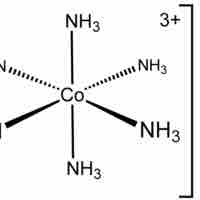
Octahedral complexes have six ligands symmetrically arranged around a central atom, defining the vertices of an octahedron.
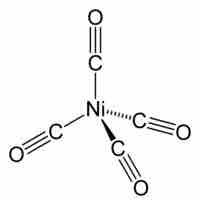
Both tetrahedral and square planar complexes have a central atom with four substituents.

Transition metal complexes are often colored due to either d-d or change band electron transitions induced by the absorption of light.
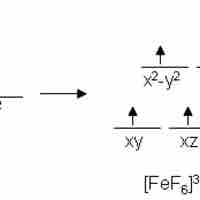
Metal complexes that have unpaired electrons are magnetic.

Coordination complexes are anionic ligands bound to a cationic metal.

Extractive metallurgy is the study of the processes used in the separation and concentration of raw materials.
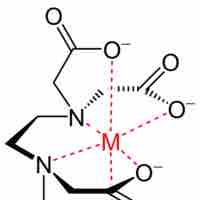
Chelating agents are ligands for metals that bind via multiple atoms, thus taking up several coordination sites on the metal.
Coordination complexes and their chemistry can be used to analyze the composition of a solution by precipitation or colorimetric analysis.
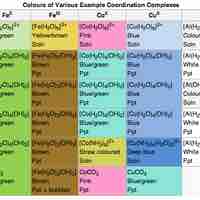
The electronic configuration of some metal complexes gives them important properties, such as color in coordination compounds.
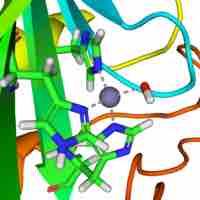
Coordination complexes are found in many biomolecules, especially as essential ingredients for the active site of enzymes.