In chemistry, a coordination or metal complex consists of an atom or ion (usually metallic) and a surrounding array of bound molecules or anions known as ligands or complexing agents. Many metal-containing compounds consist of coordination complexes.
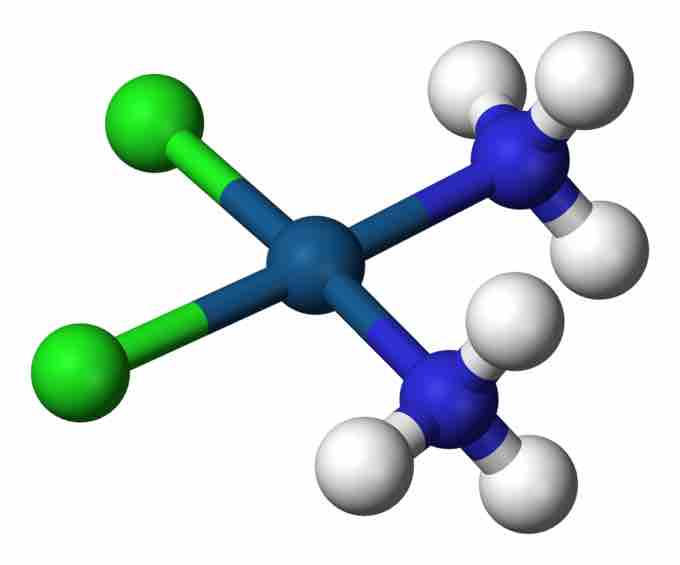
Cisplatin
This complex, PtCl2(NH3)2, is an anti-tumor drug and an example of a coordination complex.
Structure of Coordination Complexes
Donor Atom
The atom within a ligand that is bonded to the central atom or ion is called the donor atom. A typical complex is bound to several donor atoms, which can be same or different elements.
Polydentate (multiple bonded) ligands consist of several donor atoms, several of which are bound to the central atom or ion. These complexes are called chelate complexes, the formation of which is called chelation, complexation, and coordination.
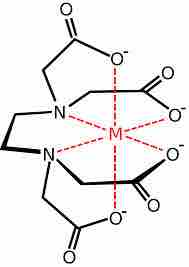
EDTA chelation
The EDTA molecule has six different donor atoms that form the complex.
Ligands
The ions or molecules surrounding the central atom are called ligands. These are generally bound to the central atom by a coordinate covalent bond (donating electrons from a lone electron pair into an empty metal orbital). There are also organic ligands such as alkenes whose pi (π) bonds can coordinate to empty metal orbitals. An example is ethene in the complex known as Zeise's salt, K+[PtCl3(C2H4)]−.
The central atom or ion, together with all ligands, comprise the coordination sphere. The central atoms or ion and the donor atoms comprise the first coordination sphere. Coordination refers to the coordinate covalent bonds (dipolar bonds) between the ligands and the central atom.
Originally, a complex implied a reversible association of molecules, atoms, or ions through such weak chemical bonds. As applied to coordination chemistry, this meaning has evolved. Some metal complexes are formed virtually irreversibly and many are bound together by bonds that are quite strong.
Reactivity
Electron Transfers
A common reaction between coordination complexes involving ligands are electron transfers. There are two different mechanisms of electron transfer redox reactions: inner sphere or outer sphere electron transfer. In electron transfer, an electron moves from one atom to another, changing the charge on each but leaving the net charge of the system the same.
Ligand Exchange
One important indicator of reactivity is the rate of degenerate exchange of ligands. For example, the rate of interchange of the coordinate water in [M(H2O)6]n+ complexes varies over 20 orders of magnitude. Complexes where the ligands are released and rebound rapidly are classified as labile. Such labile complexes can be quite stable thermodynamically. Typically they either have low-charge (Na+), electrons in d orbitals that are antibonding with respect to the ligands (Zn2+), or lack covalency (Ln3+, where Ln is any lanthanide).
The lability of a metal complex also depends on the high-spin vs. low-spin configurations when such is possible. Thus, high-spin Fe(II) and Co(III) form labile complexes, whereas low-spin analogues are inert.
Associate Processes
Complexes that have unfilled or half-filled orbitals often show the capability to react with substrates. Most substrates have a singlet ground-state; that is, they have lone electron pairs (e.g., water, amines, ethers). These substrates need an empty orbital to be able to react with a metal center. Some substrates (e.g., molecular oxygen) have a triplet ground state. Metals with half-filled orbitals have a tendency to react with such substrates. If the ligands around the metal are carefully chosen, the metal can aid in (stoichiometric or catalytic) transformations of molecules or be used as a sensor.