Octahedral molecular geometry describes the shape of compounds wherein six atoms or groups of atoms or ligands are symmetrically arranged around a central atom. The octahedron has eight faces, hence the prefix octa-. An example of an octahedral compound is molybdenum hexacarbonyl (Mo(CO)6).
The term octahedral is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N-H bonds, is referred to as octahedral.
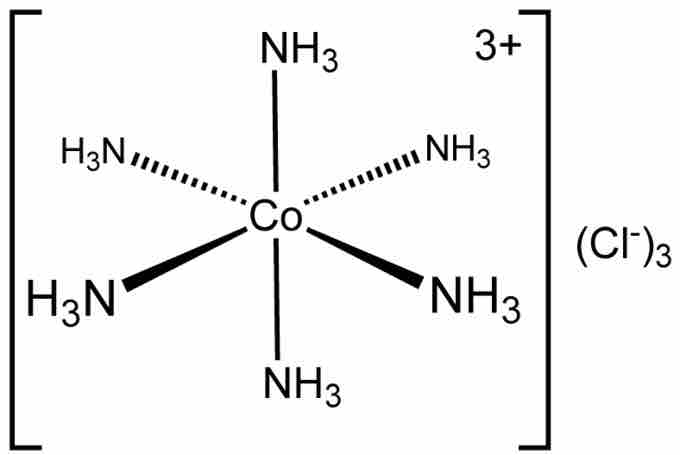
Hexamminecobalt(III) chloride
Example of an octahedral coordination complex.
When two or more types of ligands are coordinated to an octahedral metal center, the complex can exist as isomers. The number of possible isomers can reach 30 for an octahedral complex with six different ligands (in contrast, only two stereoisomers are possible for a tetrahedral complex with four different ligands).
For a free ion, such as gaseous Ni2+ or Mo, the d orbitals are degenerate. In an octahedral complex, this degeneracy is lifted. The dz2 and dx2−y2 (the so-called eg set), which are aimed directly at the ligands, are destabilized. On the other hand, the dxz, dxy, and dyz orbitals (the so-called t2g set) see a decrease in energy.
Given that such a variety of octahedral complexes exist, it is not surprising that a wide variety of reactions have been described. These reactions can be classified as follows:
- Ligand substitution reactions (via a variety of mechanisms)
- Ligand addition reactions, including protonation (among many others)
- Redox reactions (in which electrons are gained or lost)
- Rearrangements where the relative stereochemistry of the ligands change within the coordination sphere
Many reactions of octahedral transition metal complexes occur in water. For example, [Co(NH3)5Cl]2+ slowly aquates to give [Co(NH3)5(H2O)]3+ in water, especially in the presence of acid or base.