The Crystal Field Theory (CFT) is a model for the bonding interaction between transition metals and ligands. It describes the effect of the attraction between the positive charge of the metal cation and negative charge on the non-bonding electrons of the ligand. When the ligands approach the central metal ion, the degeneracy of electronic orbital states, usually d or f orbitals, are broken due to the static electric field produced by a surrounding charge distribution. CFT successfully accounts for some magnetic properties, colors, and hydration energies of transition metal complexes, but it does not attempt to describe bonding.
The electrons in the d orbitals of the central metal ion and those in the ligand repel each other due to repulsion between like charges. Therefore, the d electrons closer to the ligands will have a higher energy than those further away, which results in the d orbitals splitting in energy. This splitting is affected by:
- the nature of the metal ion
- the metal's oxidation state (a higher oxidation state leads to a larger splitting)
- the arrangement of the ligands around the metal ion
- the nature of the ligands surrounding the metal ion
All of the d orbitals have four lobes of electron density, except for the dz2 orbital, which has two opposing lobes and a doughnut of electron density around the middle. The d orbitals can also be divided into two smaller sets. The dx2-y2 and dz2 all point directly along the x, y, and z axes. They form an eg set. On the other hand, the lobes of the dxy, dxz, and dyz all line up in the quadrants, with no electron density on the axes. These three orbitals form the t2g set. In most cases, the d orbitals are degenerate, but sometimes they can split, with the eg and t2g subsets having different energy. The CFT accounts for this.
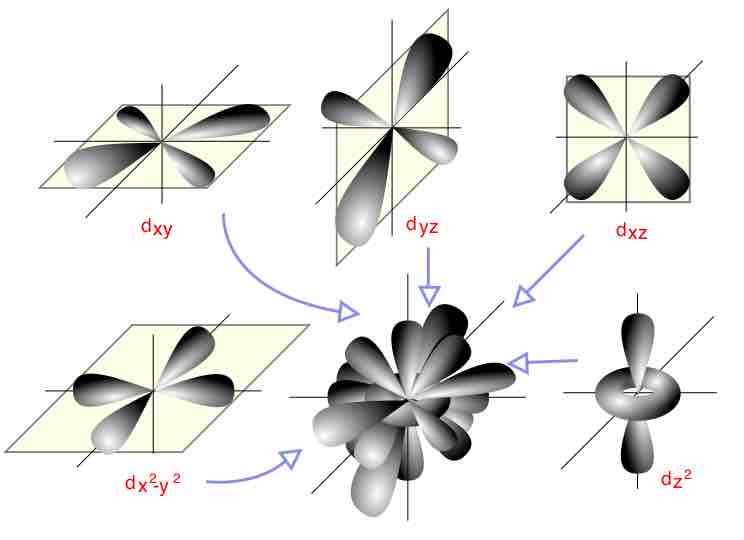
d orbitals
This gives an overview of the d orbitals. The central model shows the combined d-orbitals on one set of axes.
The crystal field stabilization energy (CFSE) is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d orbitals are split in a ligand field, some of them become lower in energy than before. For example, in the case of an octahedron, the t2g set becomes lower in energy. As a result, if there are any electrons occupying these orbitals, the metal ion is more stable in the ligand field by the amount known as the CFSE. Conversely, the eg orbitals are higher in energy. So, putting electrons in them reduces the amount of CFSE.
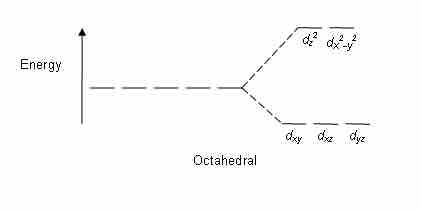
Octahedral CFT splitting
Electron diagram for octahedral d shell splitting.
Crystal field stabilization is applicable to the transition-metal complexes of all geometries. The reason that many d8 complexes are square-planar is the very large amount of crystal field stabilization that this geometry produces with this number of electrons.
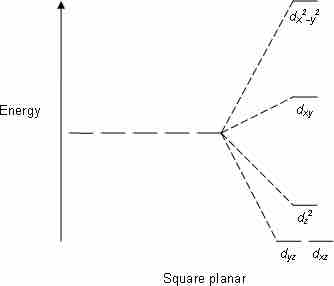
Square planar CFT splitting
Electron diagram for square planer d subshell splitting.