This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 188,661 times.
There are many ways to measure bacteria growth, and some are more complex than others. Though you sacrifice some consistency in your measurements, the simplest methods are accurate enough and used commonly. The most well-known methods are observing and counting the bacteria, measuring wet or dry mass, and measuring turbidity. Your school’s lab should have the equipment and supplies to conduct at least one of these experiments.[1]
Steps
Observing Bacteria Directly
-
1Gather your materials. There are a few special tools you should have in addition to what is stocked in most biology labs. Having your instruments and containers ahead of time will allow you to complete the experiment without having to run back and forth to the cabinet. It is important to know what each piece will be used for up front. You should know some basic terms as well.
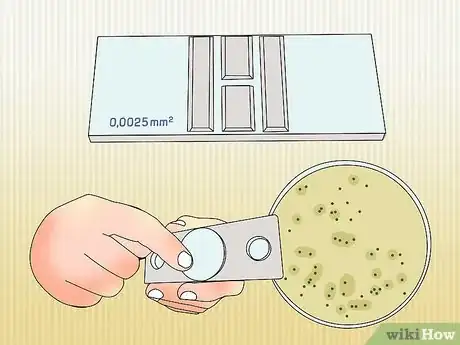
- Get a counting chamber. These tools have a chamber, slide, and microscope built in, so they are easy to set up and use. You can buy them from lab and classroom supply companies. They come with instructions that guide you through the whole process. [2]
- Get a pour plate or a spread plate. These are the containers you will observe the bacteria in.
- Culture is the term used to describe an organism grown artificially for an experiment.
- Broth is a liquid medium that the culture grows in.
-
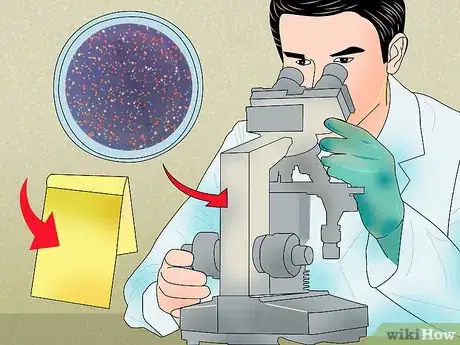
2Use your pour plate or spread plate. You can also place the bacteria directly onto a plate for viewing under a microscope. Apply the culture to the plate and place under a microscope. Observe how many bacteria cells there are.Advertisement
-
3Make sure you have the concentration right. If there are too many bacteria, they will overlap and you won’t get an accurate count. Dilute the culture by mixing it with more broth. If there are too few, you won’t have enough for an accurate number. Filter out the broth using a filtration system, or add more of the culture if you have it available to you.
-
4Count the bacteria. The last step is physically counting them. Look through the magnifier on the counting chamber and write down how many you see. Compare this with other tests.
Measuring Wet and Dry Mass
-
1Make sure you have the right equipment. This involves the use of expensive equipment and is time-consuming. Unless your lab is stocked with the right equipment, consider trying a different method. However, if you can, measuring wet and dry mass gives you more consistent readings. You will need:[3]
- Hydraulic gravity convection oven
- Aluminum weighing pan
- Set of flasks
- Centrifuge or a laboratory filtration system
-
2Make sure your culture is in a flask. If not, pour it into one. It should be in broth at this stage, though it will be separated out again later.
-
3Dry an aluminum weighing pan in your lab oven. An alternative is a cellulose acetate filter membrane, 47mm in diameter, 0.45µm in pore size.[4] Whichever you use, weigh it prior to drying so you know how much to subtract from your measurement later when you have the bacteria cells on it.
-
4Stir the flask you put your culture in so that it is mixed evenly. The cells will naturally sink due to gravity. Give them a stir to make sure they are evenly spread out in the flask. This way you will get a more representative sample.
-
5Use a centrifuge to separate the bacteria cells from the broth. This tool spins the flask and a counterweight rapidly, draining the broth and leaving the culture. See this guide for a more detailed description of the process.
-
6Scrape out the paste and put it on the weighing pan. Discard the broth. You won't need it any more, but you will need the flask, so hold onto it.
-
7Rinse the centrifuge and pour the rinse water in the pan. Add the flask of rinse water to the bacteria cells. This gives you wet weight.
-
8Find the dry weight. To measure dry weight, place the pan in the oven and dry at 100ºC for 6 to 24 hours, following the instructions of your specific oven and/or weighing pan. Make sure you are not over this temperature so the bacteria don't burn. Weigh the bacteria after the drying process is complete, and remember to subtract the weight of the weighing pan to get the dry weight.[5]
Measuring Turbidity
-
1Get the necessary equipment. You will need a light source and a spectrophotometer. These are available in science lab supply stores. The spectrophotometer will have instructions for the proper usage of the model you bought. This equipment is inexpensive and easy to use, so this is one of the more common methods for measuring bacterial growth.[6]
-
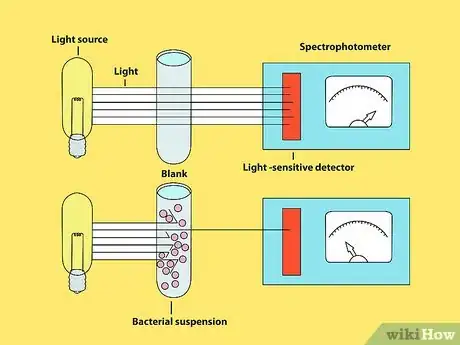
2Shine the light through the sample. In layman’s terms, turbidity is how cloudy your sample is. You should get a turbidity reading, which is measured in NTU (Nephelometric Turbidity Units). Your spectrophotometer may take some calibration before you can accurately measure the turbidity of your sample, so consult the manufacturer's reference manual prior to use.
-
3Make some notes. Turbidity corresponds to the amount of bacteria in the sample. The spectrophotometer will also tell you the percent transmission (%T). This number will be higher if the turbidity is lower. Compare your numbers from different tests to measure bacterial growth.
Community Q&A
-
QuestionWhy do we measure bacterial growth? What is the importance of measurement of bacterial growth?
 Community AnswerIt helps in nature, medicine and various other industries. In nature, bacteria help to fix nitrogen in plants and enhance soil fertility, digestion of cellulose and sewage treatment. In medicine, they use bacteria to manufacture drugs and vitamin supplements. Other industries use bacteria for baking, preparation of alcoholic drinks, making of cheese and yogurt and tanning of hides and skin.
Community AnswerIt helps in nature, medicine and various other industries. In nature, bacteria help to fix nitrogen in plants and enhance soil fertility, digestion of cellulose and sewage treatment. In medicine, they use bacteria to manufacture drugs and vitamin supplements. Other industries use bacteria for baking, preparation of alcoholic drinks, making of cheese and yogurt and tanning of hides and skin. -
QuestionWhat unit of measurement is used to count bacteria?
 Community AnswerThe unit of cell number depends on the used method. If the bacteria are counted on a growth plate, the most used unit of cell number is colony forming units per milliliter (cfu / ml). If measured by turbidity, there is no unit unless the cfu / ml could be calculated by the measurement of control probes.
Community AnswerThe unit of cell number depends on the used method. If the bacteria are counted on a growth plate, the most used unit of cell number is colony forming units per milliliter (cfu / ml). If measured by turbidity, there is no unit unless the cfu / ml could be calculated by the measurement of control probes. -
QuestionHow can I tell if the bacteria has died?
 Community AnswerBacteria is an extremely dynamic organism, and it grows exponentially in short periods of time. If you cannot detect motion or growth for a very extended period of time, and/or if the environment it's put in is hostile to the specific strain of bacteria, then it probably has died.
Community AnswerBacteria is an extremely dynamic organism, and it grows exponentially in short periods of time. If you cannot detect motion or growth for a very extended period of time, and/or if the environment it's put in is hostile to the specific strain of bacteria, then it probably has died.
Warnings
- Because you are dealing with colonies of bacteria, take the necessary precautions such as wearing safety glasses and gloves. You may also want to wear a dust mask, especially if you are not sure of the type of bacteria you are culturing.⧼thumbs_response⧽
- Take precautions with any type of bacteria, even if you consider it to be harmless, by ensuring that any open sores, cuts and scratches are completely covered before you begin.⧼thumbs_response⧽
Things You'll Need
- Bacterial broth, tap water, swimming pool water, or pond water
- Sterile medium such as agar
- Petri dishes
- Sterile pipettes
- Microscope (optional)
- Counting chamber
- Latex gloves
- Safety glasses
- Hydraulic oven
References
About This Article
The easiest way to measure bacterial growth is to put your sample on a clear glass plate under a microscope and count how many bacteria cells there are. Alternatively, you can measure turbidity, which is the amount of bacteria in your sample. To do that, first get a light source and a spectrophotometer, which are cheap and easy to use, from a science lab supply store. Then, shine the light through the sample to the spectrophotometer, which will give you the sample’s turbidity reading. To learn how to measure bacterial growth by measuring wet and dry mass, scroll down!