Chapter 2
The Chemical Foundation of Life
By Boundless
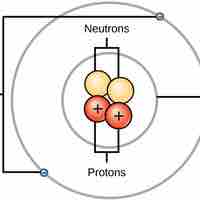
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
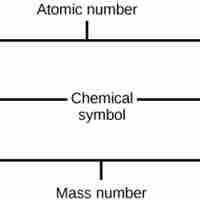
The atomic number is the number of protons in an element, while the mass number is the number of protons plus the number of neutrons.

Isotopes are various forms of an element that have the same number of protons, but a different number of neutrons.
Everything in the universe is made of one or more elements. The periodic table is a means of organizing the various elements according to similar physical and chemical properties.
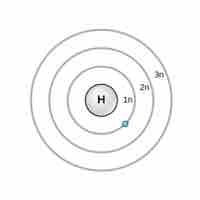
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells.
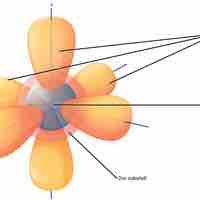
Electron orbitals are three-dimensional representations of the space in which an electron is likely to be found.
Chemical reactions occur when two or more atoms bond together to form molecules or when bonded atoms are broken apart.
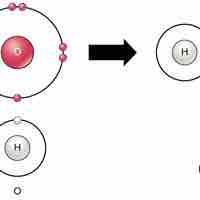
Ionic bonds are attractions between oppositely charged atoms or groups of atoms where electrons are donated and accepted.
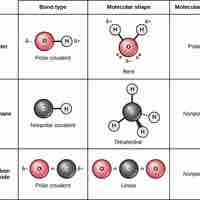
Covalent bonds result from a sharing of electrons between two atoms and hold most biomolecules together.
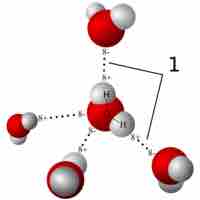
Hydrogen bonds and van der Waals interactions are two types of weak bonds that are necessary to the basic building blocks of life.

Water's polarity is responsible for many of its properties including its attractiveness to other molecules.
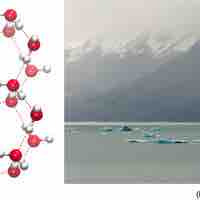
The orientation of hydrogen bonds as water changes states dictates the properties of water in its gaseous, liquid, and solid forms.
Water is able to absorb a high amount of heat before increasing in temperature, allowing humans to maintain body temperature.
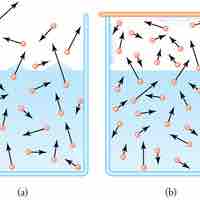
Evaporation of water requires a substantial amount of energy due to the high heat of vaporization of water.
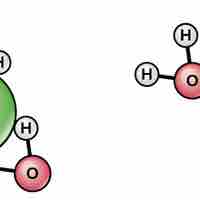
Water's polarity makes it an excellent solvent for other polar molecules and ions.

Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules.
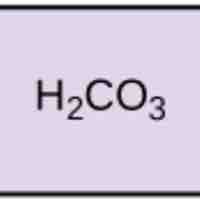
Acids dissociate into H+ and lower pH, while bases dissociate into OH- and raise pH; buffers can absorb these excess ions to maintain pH.
Carbon is the most important element to living things because it can form many different kinds of bonds and form essential compounds.
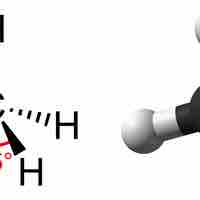
Hydrocarbons are important molecules that can form chains and rings due to the bonding patterns of carbon atoms.
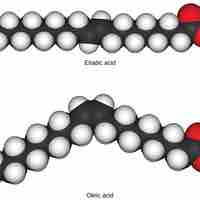
Isomers are molecules with the same chemical formula but have different structures, which creates different properties in the molecules.
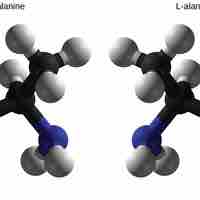
Enantiomers share the same chemical structure and bonds but differ in the placement of atoms such that they are mirror images of each other.
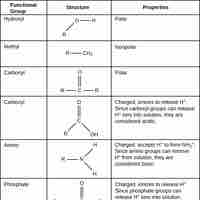
Functional groups are groups of molecules attached to organic molecules and give them specific identities or functions.