The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds are known as isomers.
Structural Isomers
Structural isomers (such as butane and isobutane ) differ in the placement of their covalent bonds. Both molecules have four carbons and ten hydrogens (C4H10), but the different arrangement of the atoms within the molecules leads to differences in their chemical properties. For example, due to their different chemical properties, butane is suited for use as a fuel for cigarette lighters and torches, whereas isobutane is suited for use as a refrigerant and a propellant in spray cans.
Geometric Isomers
Geometric isomers, on the other hand, have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms, especially in carbon-to-carbon double bonds. In the simple molecule butene (C4H8), the two methyl groups (CH3) can be on either side of the double covalent bond central to the molecule. When the carbons are bound on the same side of the double bond, this is the cis configuration; if they are on opposite sides of the double bond, it is a trans configuration. In the trans configuration, the carbons form a more or less linear structure, whereas the carbons in the cis configuration make a bend (change in direction) of the carbon backbone.
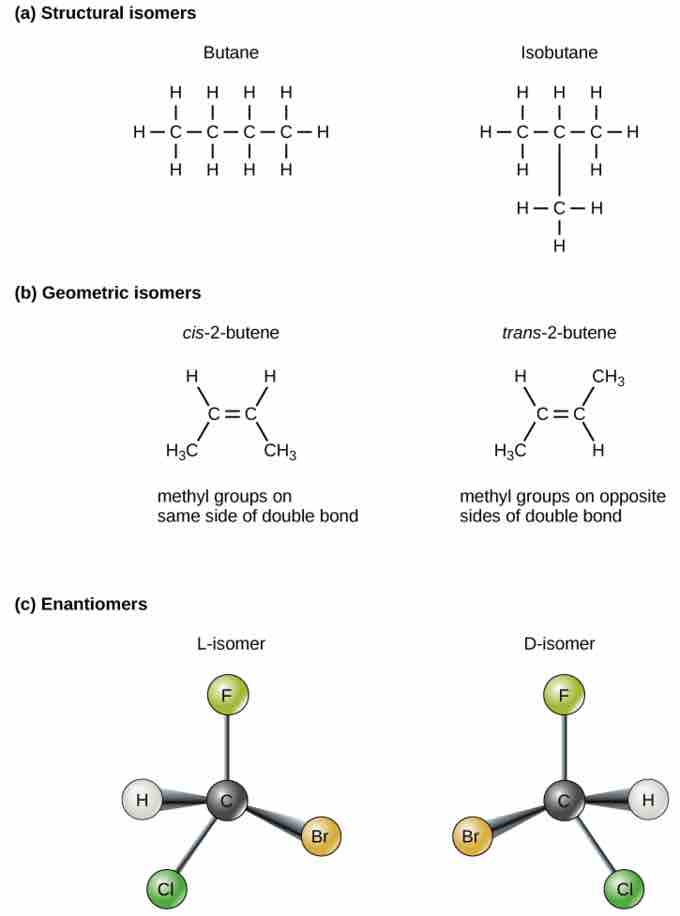
Isomers
Molecules that have the same number and type of atoms arranged differently are called isomers. (a) Structural isomers have a different covalent arrangement of atoms. (b) Geometric isomers have a different arrangement of atoms around a double bond. (c) Enantiomers are mirror images of each other.
Cis or Trans Configurations
In triglycerides (fats and oils), long carbon chains known as fatty acids may contain double bonds, which can be in either the cis or trans configuration . Fats with at least one double bond between carbon atoms are unsaturated fats. When some of these bonds are in the cis configuration, the resulting bend in the carbon backbone of the chain means that triglyceride molecules cannot pack tightly, so they remain liquid (oil) at room temperature. On the other hand, triglycerides with trans double bonds (popularly called trans fats), have relatively linear fatty acids that are able to pack tightly together at room temperature and form solid fats.
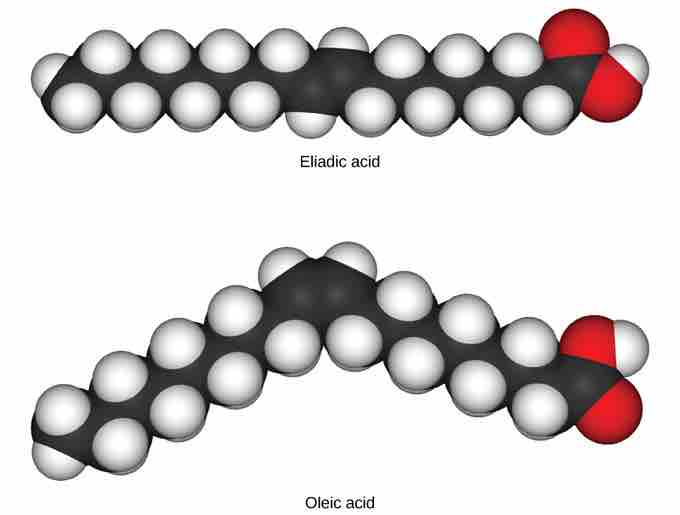
Cis and Trans Fatty Acids
These space-filling models show a cis (oleic acid) and a trans (eliadic acid) fatty acid. Notice the bend in the molecule cause by the cis configuration.
In the human diet, trans fats are linked to an increased risk of cardiovascular disease, so many food manufacturers have reduced or eliminated their use in recent years. In contrast to unsaturated fats, triglycerides without double bonds between carbon atoms are called saturated fats, meaning that they contain all the hydrogen atoms available. Saturated fats are a solid at room temperature and usually of animal origin.