Water's Heat of Vaporization
Water in its liquid form has an unusually high boiling point temperature, a value close to 100°C. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water has a heat of vaporization value of 40.65 kJ/mol. A considerable amount of heat energy (586 calories) is required to accomplish this change in water. This process occurs on the surface of water. As liquid water heats up, hydrogen bonding makes it difficult to separate the water molecules from each other, which is required for it to enter its gaseous phase (steam). As a result, water acts as a heat sink, or heat reservoir, and requires much more heat to boil than does a liquid such as ethanol (grain alcohol), whose hydrogen bonding with other ethanol molecules is weaker than water's hydrogen bonding. Eventually, as water reaches its boiling point of 100° Celsius (212° Fahrenheit), the heat is able to break the hydrogen bonds between the water molecules, and the kinetic energy (motion) between the water molecules allows them to escape from the liquid as a gas. Even when below its boiling point, water's individual molecules acquire enough energy from each other such that some surface water molecules can escape and vaporize; this process is known as evaporation .
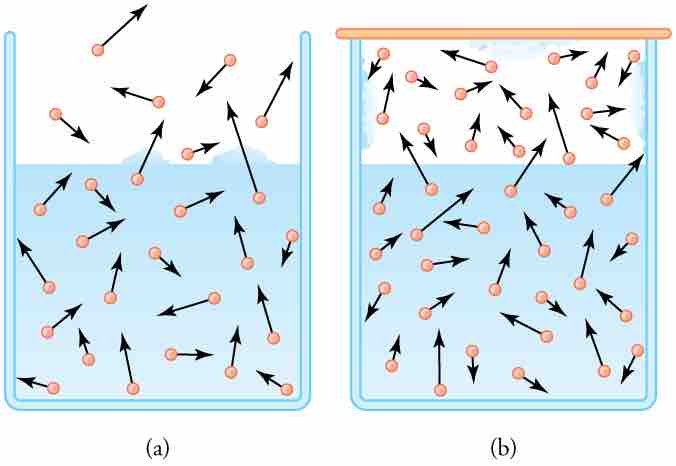
Humidity, Evaporation, and Boiling
(a) Because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. (b) If the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. This vapor density and the partial pressure it creates are the saturation values. They increase with temperature and are independent of the presence of other gases, such as air. They depend only on the vapor pressure of water.
The fact that hydrogen bonds need to be broken for water to evaporate means that a substantial amount of energy is used in the process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place. In many living organisms, including humans, the evaporation of sweat, which is 90 percent water, allows the organism to cool so that homeostasis of body temperature can be maintained.