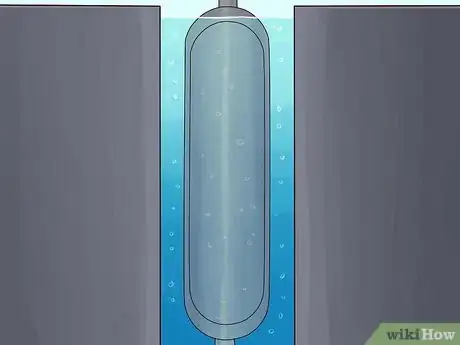wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 14 people, some anonymous, worked to edit and improve it over time.
There are 10 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 95% of readers who voted found the article helpful, earning it our reader-approved status.
This article has been viewed 57,826 times.
Learn more...
Uranium is used as a power source in nuclear reactors and was used to make the first atomic bomb, dropped on Hiroshima in 1945.[1] Uranium is mined as an ore called pitchblende,[2] and consists of several isotopes of different atomic weights and different levels of radioactivity. To be used in fission reactions, the amount of the 235U isotope must be increased to a level to permit ready fission in a reactor or bomb. This process is called enriching uranium, and there are several ways to do it.
Steps
The Basic Enrichment Process
-
1Decide what the uranium will be used for. Most mined uranium contains only about 0.7 percent 235U, with most of the rest being the comparatively stable isotope 238U.[3] What type of fission reaction the uranium will be used for determines what the level of 235U must be raised to for the uranium to be used effectively.
- Uranium used in most nuclear power plants needs to be enriched to a level of 3 to 5 percent 235U.[4] [5] (A few nuclear reactors, such as the CANDU reactor in Canada and the Magnox reactor in the United Kingdom, are designed to use unenriched uranium.)
- Uranium used for atomic bombs and warheads, in contrast, needs to be enriched to 90 percent 235U.
-
2Convert the uranium ore to a gas. Most of the methods currently in existence for enriching uranium require the ore to be converted to a low-temperature gas. Fluorine gas is normally pumped into an ore conversion plant; the uranium oxide gas reacts with the fluorine to produce uranium hexafluoride (UF6). The gas is then acted on to separate out and gather the 235U isotope.Advertisement
-
3Enrich the uranium. The remaining sections of this article describe the various processes available to enrich uranium. Of these, gaseous diffusion and gas centrifuge are the two most common, but the laser isotope separation process is expected to replace them.[6]
-
4Convert the UF6 gas to uranium dioxide (UO2). Once enriched, the uranium needs to be converted to a stable solid form for its intended use.
- Uranium dioxide used as fuel in nuclear reactors is made into centered ceramic pellets encased in metal tubes to make 4m (13.12-foot) long rods
Gaseous Diffusion Process
-
1Pump UF6 through pipelines.
-
2Force the gas through a porous filter or membrane. Because the 235U isotope is lighter than the 238U isotope, UF6 containing the lighter isotope will diffuse through the membrane faster than the heavier isotope.
-
3Repeat the diffusion process until enough 235U is collected. The repeated diffusion is called a cascade. It may take as many as 1,400 passes through porous membranes to get enough 235U to enrich the uranium sufficiently.
-
4Condense the UF6 gas into liquid form. Once the gas is sufficiently enriched, it is condensed into a liquid and then stored in containers, where it cools and solidifies for transport to be made into fuel pellets.
- Because of the number of passes required, this process is energy-intensive and is being phased out. In the United States, only one gaseous diffusion enrichment plant remains, located in Paducah, Kentucky.[7]
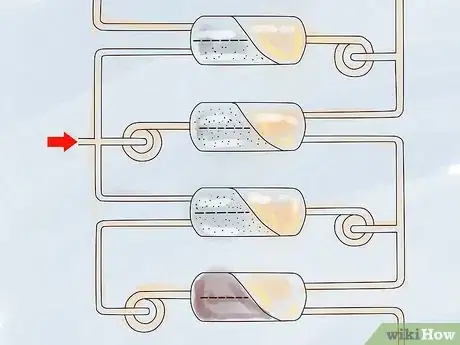
Gas Centrifuge Process
-
1Assemble a number of high-speed rotating cylinders. These cylinders are the centrifuges. The centrifuges are assembled in both series and parallel layouts.
-
2Pipe the UF6 gas into the centrifuges. The centrifuges use centripetal acceleration to send the heavier 238U-bearing gas to the cylinder wall and the lighter 235U-bearing gas to the center.
-
3Extract the separated gases.
-
4Reprocess the separated gases in separate centrifuges. The 235U-rich gases are sent to a centrifuge where still more 235U is extracted, while the 235U-depleted gas goes to a different centrifuge to extract still more of the remaining 235U. This enables the centrifuge process to extract much more 235U than the gaseous diffusion process can.[8]
- The gas centrifuge process was first developed in the 1940s, but was not brought into significant use until the 1960s, when its lower energy requirements for producing enriched uranium became important. At present, a gas centrifuge processing plant exists in the United States in Eunice, New Mexico.[9] In contrast, Russia currently has four such plants, Japan and China have two each, while the United Kingdom, the Netherlands, and Germany each have one.
Areodynamic Separation Process
-
1Build a series of stationary narrow cylinders.
-
2Inject UF6 gas into the cylinders at high speed. The gas is blown into the cylinders in such a way that it is induced to spin in cyclonic fashion, producing the same kind of separation between 235U and 238U as is achieved in a rotating centrifuge.
- One method being developed in South Africa injects the gas into the cylinder on a tangent. It is presently being tested with light isotopes such as those in silicon.
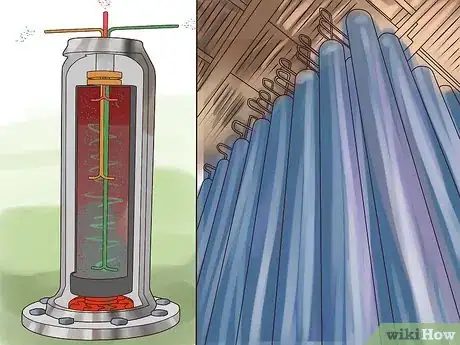
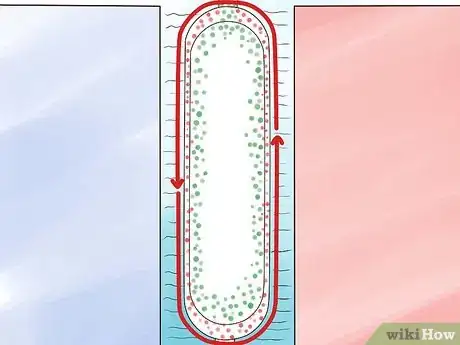
Liquid Thermal Diffusion Process
-
1Liquefy the UF6 gas under pressure.
-
2Construct a pair of concentric pipes. The pipes should be fairly tall, with taller pipes enabling more separation of the 235U and 238U isotopes.
-
3Surround the pipes with a jacket of liquid water. This will cool the outer pipe.
-
4Pump the liquid UF6 between the pipes.
-
5Heat the inner pipe with steam. The heat will create a convection current in the UF6 that will draw the lighter 235U isotope toward the hotter inner pipe and push the heavier 238U isotope toward the colder outer pipe.
Electromagnetic Isotope Separation Process
-
1Ionize the UF6 gas.
-
2Pass the gas through a strong magnetic field.
-
3Separate the ionized uranium isotopes by the trails they leave when passing through the magnetic field. Ions of 235U leave trails that curve differently than those of 238U. These ions can be isolated to enrich uranium.
- This method was used to process uranium for the atomic bomb dropped on Hiroshima in 1945 and was also the enrichment method Iraq used in its nuclear weapons program of 1992. It requires 10 times more energy than gaseous diffusion, making it impractical for large-scale enrichment programs.
Laser Isotope Separation Process
-
1Tune a laser to a specific color. The laser light needs to be entirely of a specific wavelength (monochromatic). This wavelength will target only 235U atoms, while leaving the 238U atoms untouched.
-
2Shine the laser light on the uranium. Unlike the other uranium enrichment processes, you don’t have to use uranium hexafluoride gas, although most of the laser processes do. You can also use an alloy of uranium and iron as the uranium source, which the Atomic Vapor Laser Isotope Separation (AVLIS) process does.
-
3Extract the uranium atoms with excited electrons. These will be atoms of 235U.
Warnings
- Enriched uranium can ordinarily be reprocessed only once.⧼thumbs_response⧽
- Reprocessed uranium must be kept under heavy shielding, because the 232U it contains decays into elements that emit a lot of gamma radiation.⧼thumbs_response⧽
- Uranium is actually only weakly radioactive; however, when processed into UF6 gas, it becomes a toxic chemical that reacts with water to form corrosive hydrofluoric acid. (This acid is commonly called “etching acid” because of its use to etch glass.)[12] Uranium enrichment plants thus require the same protective measures as chemical plants that work with fluorine, which include keeping UF6 gas under low pressure most of the time and using extra containment levels in areas requiring higher pressure.⧼thumbs_response⧽
References
- ↑ http://www.atomicheritage.org/history/science-behind-atom-bomb
- ↑ http://www.britannica.com/EBchecked/topic/462007/pitchblende
- ↑ https://www.orano.group/en/unpacking-nuclear/all-about-uranium
- ↑ http://www.cnn.com/2013/11/20/world/gallery/uranium-enrichment-explainer/
- ↑ https://tutorials.nti.org/nuclear-101/uranium-enrichment/
- ↑ http://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html
- ↑ http://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html
- ↑ http://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html
- ↑ http://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html
About This Article
To enrich uranium, introduce fluorine gas to convert the ore to uranium hexafluoride. From there, use gaseous diffusion or gas centrifuges to separate the gas so that the desired uranium isotope can be gathered. To use gaseous diffusion, pump uranium hexafluoride through pipelines, then force the gas through a porous filter to separate the desired isotope. To use the gas centrifuge process, assemble several high-speed rotating cylinders and pipe the uranium hexafluoride gas into them to separate and extract the desired uranium isotope. If you want to learn how to enrich uranium through other processes, such as aerodynamic separation or laser isotope separation, keep reading the article!