In physics and chemistry there are many conservation laws—among them, the Law of Conservation of Nucleon Number, which states that the total number of nucleons (nuclear particles, specifically protons and neutrons) cannot change by any nuclear reaction.
Radioactive Decay
Consider the three modes of decay. In gamma decay, an excited nucleus releases gamma rays, but its proton (Z) and neutron (A-Z) count remain the same:
In beta decay, a nucleus releases energy and either an electron or a positron. In the case of an electron being released, atomic mass (A) remains the same as a neutron is converted into a proton, raising atomic number by 1:
In the case of a positron being released, atomic mass remains constant as a proton is converted to a neutron, lowering atomic number by 1:
Electron capture has the same effect on the number of protons and neutrons in a nucleus as positron emission.
Alpha decay is the only type of radioactive decay that results in an appreciable change in an atom's atomic mass. However, rather than being destroyed, the two protons and two neutrons an atom loses in alpha decay are released as a helium nucleus.
Nuclear Fission
Chain reactions of nuclear fission release a tremendous amount of energy, but follow the Law of Conservation of Nucleon Number. Consider, for example, the multistep reaction that occurs when a U-235 nucleus accepts a neutron, as in :
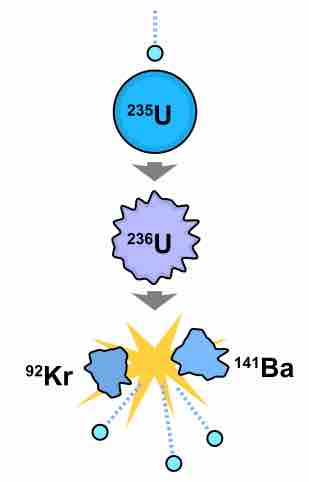
Nuclear fission of U-235
If U-235 is bombarded with a neutron (light blue small circe), the resulting U-236 produced is unstable and undergoes fission. The resulting elements (shown here as Kr-92 and Ba-141) do not contain as many nucleons as U-236, with the remaining three neutrons being released as high-energy particles, able to bombard another U-235 atom and maintain a chain reaction.
In each step, the total atomic mass of all species is a constant value of 236. This is the same with all fission reactions.
Nuclear Fusion
Finally, nuclear fusion follows the Law of Conservation of Nucleon Number. Consider the fusion of deuterium and tritium (both hydrogen isotopes):
It is well understood that the tremendous amounts of energy released by nuclear fission and fusion can be attributed to the conversion of mass to energy. However, the mass that is converted to energy is rather small compared to any sample, and never includes the conversion of a proton or neutron to energy. Thus, the number of nucleons before and after fission and fusion is always constant.