A solution is defined as a homogeneous mixture of both a solute and solvent. Solutions generally have different properties than the solvent and solute molecules that compose them. Some special properties of solutions are dependent solely on the amount of dissolved solute molecules, regardless of what that solute is; these properties are known as colligative properties.
Osmosis is defined as the net flow or movement of solvent molecules through a semipermeable membrane through which solute molecules cannot pass. If a solution consisting of both solute and solvent molecules is placed on one side of a membrane and pure solvent is placed on the other side, there is a net flow of solvent into the solution side of the membrane.
Imagine osmosis taking place in an upright U-tube. The height of the solution will continue to increase due to a net flow of solvent until the added pressure of the height will cause the flow of solution to stop. The height difference between the two sides can be be converted into pressure to find the osmotic pressure exerted on the solution by the pure solvent.
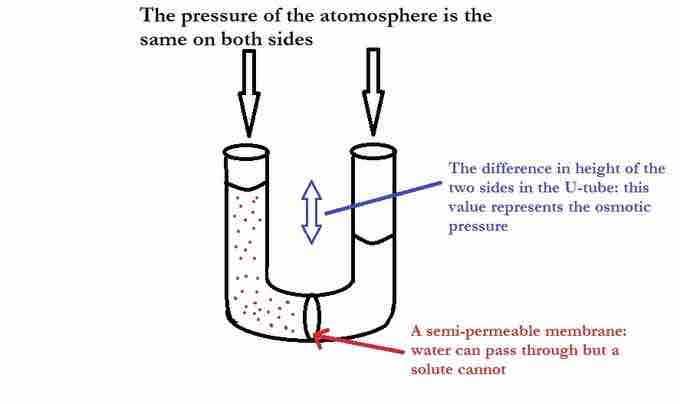
U-Tube showing osmotic pressure
On the left side of the U-tube is an aqueous solution, and on the right side is pure water. The pure water is trying to dilute the solution by travelling through the semipermeable membrane. Eventually the added weight of the extra water on the left causes enough pressure to stop osmosis.
Osmotic pressure is the pressure that needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane. Osmotic pressure can also be explained as the pressure necessary to nullify osmosis. One way to stop osmosis is to increase the hydrostatic pressure on the solution side of the membrane; this ultimately squeezes the solvent molecules closer together, increasing their "escaping tendency." The escaping tendency of the solution can be raised until it eventually equals that of the molecules in the pure solvent; at this point, osmosis will cease. The osmotic pressure is the pressure required to achieve osmotic equilibrium.
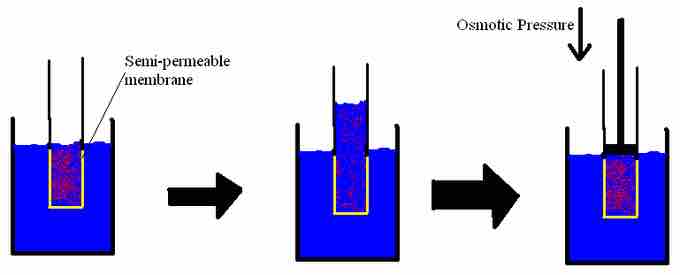
Osmotic pressure
Osmotic pressure is the pressure required to stop osmosis.
The osmotic pressure (II) of an ideal solution can be approximated by the Morse equation:
Here, i is the van 't Hoff factor, M is the molarity of the solution, R is the gas constant, and T is the absolute temperature in Kelvin. We can see from this equation that the amount of solute present in the solution will directly affect the osmotic pressure of the system.
Example
What is the osmotic pressure of a 1.35 M solution of NaCl at 25 oC?
First, fill in all of the necessary information, and then solve:
i = 2 (NaCl breaks into two particles)
M = 1.35
R = 0.0821
T = 25 oC + 273 = 298 K