Concept
Version 10
Created by Boundless
Osmotic Pressure
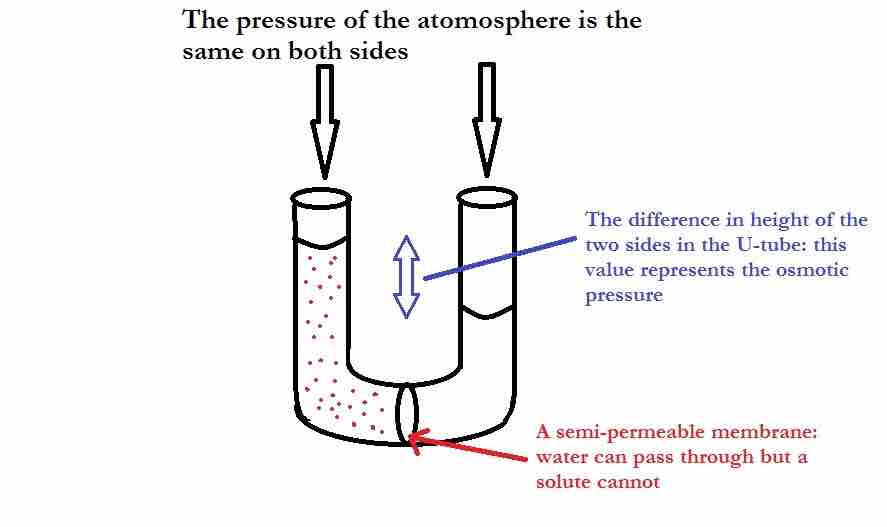
U-Tube showing osmotic pressure
On the left side of the U-tube is an aqueous solution, and on the right side is pure water. The pure water is trying to dilute the solution by travelling through the semipermeable membrane. Eventually the added weight of the extra water on the left causes enough pressure to stop osmosis.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: