Electrolyte Solutions
A simple example of an electrolyte solution is sodium chloride in water. In the presence of water, solid sodium chloride dissociates as it is dissolved, forming an electrolyte solution:
Nonelectrolyte solutions are those in which the solute does not dissociate into ions when dissolved; sugar does not dissociate, for example. The number of moles of dissolved particles is greater for electrolyte solutions, so there will be a greater impact on colligative properties.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phase, either liquid or solid, at a particular temperature. Basically, it is a measure of how much the solvent molecules tend to escape from a liquid or solid phase into the atmosphere. Vapor pressure of a liquid is a colligative property.
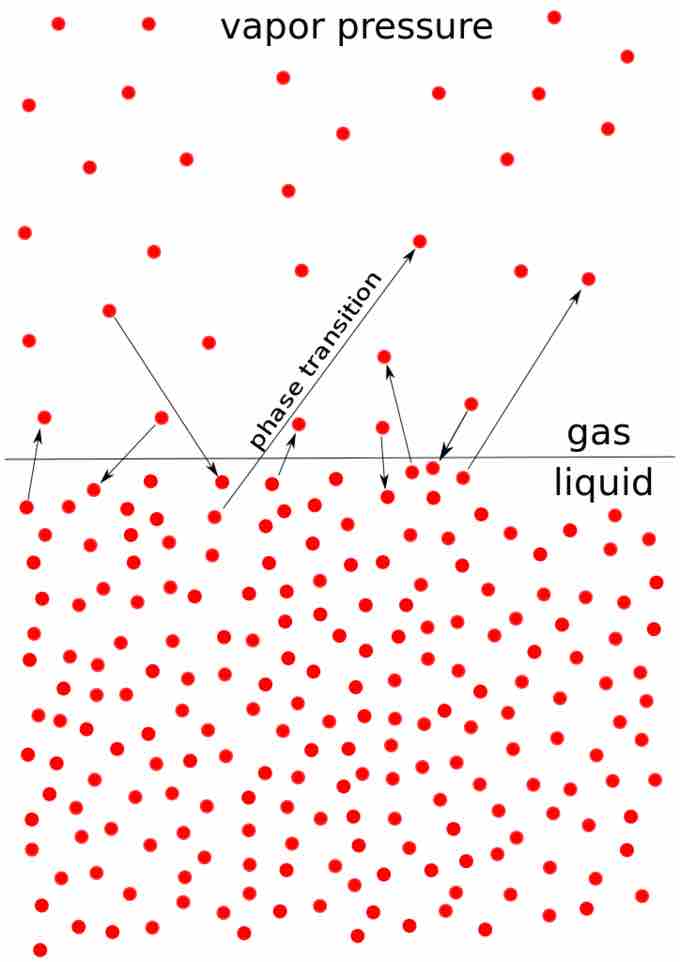
Vapor pressure
The picture shows the transition, as a result of the vapor pressure, from particles in liquid phase to gas phase and vice-versa.
To better visualize the effect of solute on the vapor pressure of a solution, consider a pure solvent. This pure solvent has a certain vapor pressure associated with it. Subjected to temperatures below the solvent's boiling point, the molecules going into the gaseous phase are mostly situated on the top layer of the solution. Now consider a solution composed of both solvent and solute. Some solute molecules will occupy space near the surface of the liquid, decreasing the number of solvent molecules that can be there. Therefore, fewer molecules are changing from the liquid phase into the gas phase, and the vapor pressure of the solvent decreases.
In an electrolyte solution, the number of dissolved particles is larger because the solute breaks apart into ions. The greater the number of ions, the larger the impact on colligative properties will be.
Example
Which would have the lowest vapor pressure at 25 oC?
a) 0.1 M solution of NaCl
b) 0.1 M solution of C6H12O6 (glucose)
c) 0.1 M solution of Al(NO3)3
The correct answer is c. All of the solutions have the same concentration, but when NaCl dissolves, it breaks into 2 particles. Glucose is a non-electrolyte and does not break apart. When Al(NO3)3 dissolves, it produces 4 particles in solution (1 Al3+ and 3 NO3-), and will have the greatest impact on the vapor pressure.