This article was co-authored by Andrew Carberry, MPH. Andrew Carberry is a Food Systems Expert and the Senior Program Associate at the Wallace Centere at Winrock International in Little Rock, Arkansas. He has worked in food systems since 2008 and has experience working on farm-to-school projects, food safety programs, and working with local and state coalitions in Arkansas. He is a graduate of the College of William and Mary and holds a Masters degree in public health and nutrition from the University of Tennessee.
There are 13 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 13 testimonials and 80% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 353,567 times.
In chemistry, pH is a measure of how acidic or basic a substance is. The pH scale runs from 0 to 14 — a pH near 0 is extremely acidic, a pH near 14 is extremely basic, and a pH of 7 is perfectly neutral. In gardening and horticulture, the pH of the soil used for growing plants can have serious effects on the health and growth of the plant. While most plants tolerate a pH of about 6.0-7.5, some grow best under narrow pH ranges, so serious gardeners should learn the basics of soil pH management.[1] See Step 1 below to start learning how to lower your soil pH.
Steps
Performing a pH Test
-
1Test the soil pH. Before adding anything to alter the soil pH, always make sure to test your soil to see how far it is from your goal pH. You can purchase a test at your local home/garden center or take a sample to your county extension office for professional testing.
-
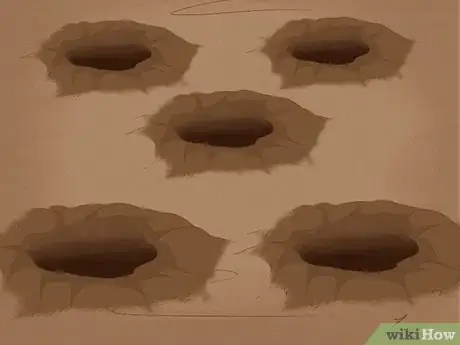
2Dig five small holes in the planting area. The pH of your garden soil is easy to determine with a commercial pH testing kit. These are usually sold at hardware stores or garden supply stores and are fairly inexpensive. To begin, you'll want to take a sample from the area you want to test. Dig five small holes (about six to eight inches deep). Select random locations within the plot — this way, you'll get a sense of the "average" pH of your soil. Don't keep any of the dirt from creating the holes.
- Note that the instructions in this section are generalized — you should use the instructions included with your specific pH testing kit.
Advertisement -
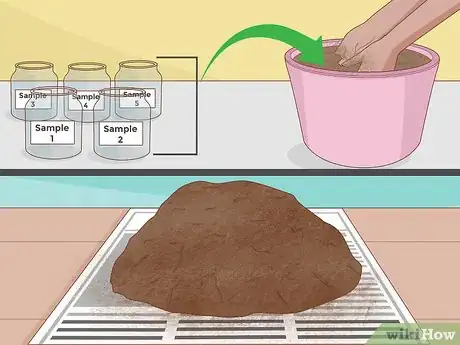
3Take a sample from each hole. Next, use your shovel or spade to take a narrow "slice" from the side of each hole. This slice should be crescent-shaped and about 1⁄2 inch (1.3 cm) thick. Try to make your sample about the same size for each hole. Add your samples to a single clean, dry bucket.
- Try to gather enough dirt in each sample that you have about a pint (0.94 liters) or more in total. For most methods of testing, this is plenty.
-
4Mix the soils in the bucket and spread on newspaper to dry. Allow your soil to dry until you can no longer detect moisture.
- It's important to make sure your soil is completely dry before proceeding — moisture can cause an inaccurate pH reading.
-
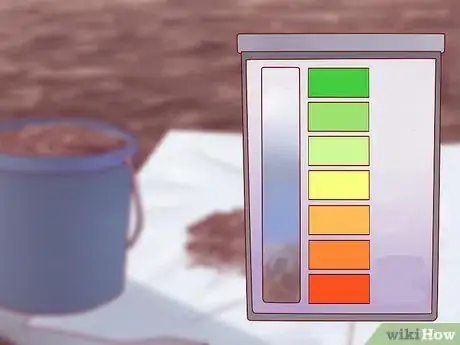
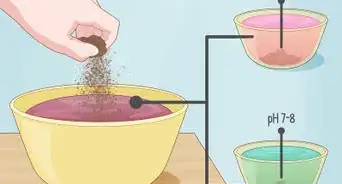
5Use your testing kit to determine the precise pH level of your soil. Depending on the type of kit you have, the method will vary. For many of the most common test kits, you'll put a small amount of your soil in an included test tube, add a few drops of liquid solution, mix by shaking, and allow the mixture to settle for a few hours.[2] Eventually, the color of the solution should change — by comparing the color of the solution to a chart provided with the kit, you can determine the pH of your soil.
- Other types of kits exist as well, so be sure to use the instructions provided with your kit. For instance, some modern electronic testing kits determine a soil pH almost instantaneously via a metal probe. You can also collect a sample and take it into your county extension office for a free soil test.
Using pH-Lowering Techniques
-
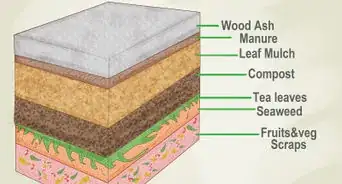
1Add organic matter. Many types of organic matter, like compost, composted manure, and acidic mulches can gradually lower your soil pH over time. As organic matter decomposes, bacteria and other microbes grow and feed upon it, creating acidic by-products in the process. Because organic matter needs time to decompose to alter the soil, this option is great for long-term goals, but won't give you dramatic results in the short-term. Many gardeners opt to add organic matter to their soil annually for a gradual, mild pH-lowering effect. However, this will not work for heavy clay soils.
- Organic matter can also give your soil other benefits — most noticeably, improving its drainage and aeration.[3]
-
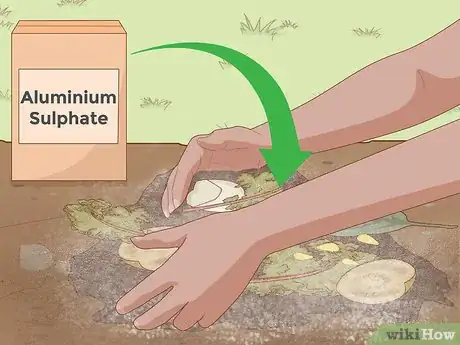
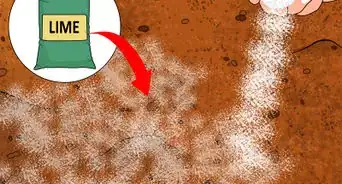
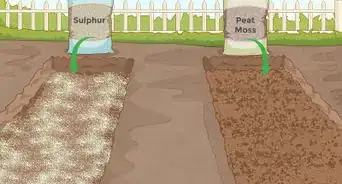
2Add aluminum sulfate. For rapid pH-lowering, don't rely on the slow, gradual decay of organic matter. Instead, use one of the many acidic soil additives available at your local garden supply store. Of these additives, aluminum sulfate, is one of the quickest-acting options available. Aluminum sulfate produces acidity in the soil as soon as it dissolves, which, for gardening purposes, means that it basically works instantly.[4] Because of this, aluminum sulfate is a great choice for urgent pH lowering.
- Depending on the starting pH of your soil, the amount of aluminum sulfate you should use can vary significantly. In very general terms, you should expect to use about 1.2 pounds of aluminum sulfate to lower the pH of a 10-square foot patch of soil by one on the pH scale (e.g., from 7.0 to 6.0, from 6.0 to 5.0, etc.).[5] However, using too much additive can be harmful to your plant, so consult an online resource (like the one here) for more precise usage information.
- Do not use aluminum sulfate for large applications because it can lead to aluminum accumulation and aluminum toxicity in soil.
-
3Add sulfur. Another useful pH-lowering soil additive is sublimed sulfur. Compared to aluminum sulfate, sulfur is generally cheaper, more powerful (in terms of the amount needed) and slower-acting. This is because sulfur needs to be metabolized by bacteria in the soil to be converted to sulfuric acid, which takes time.[6] Depending on the moisture of the soil, the amount of bacteria present, and the temperature, sulfur can take up to several months to produce a noticeable effect in the soil.
- As noted above, compared to aluminum sulfate, you will generally need a relatively small amount of pure, sublimed sulfur to produce an equivalent pH change. In general, you'll need about 0.2 pounds of sulfur to lower the pH of a 10-square foot patch of soil by one whole number on the pH scale.[7] Consult an online resource (like the one here) for more precise usage information.
-
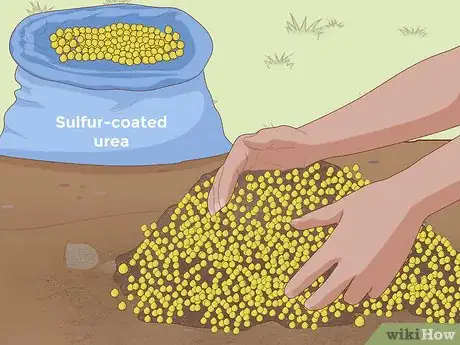
4Add sulfur-coated urea. Like sulfur and aluminum sulfate, soil additives containing sulfur-coated urea can increase the acidity of soil over time (lowering its pH). As an additive, urea is fairly quick-acting, producing some effects as soon as a week or two after being introduced to the soil.[8] Sulfur-coated urea is a common ingredient in many fertilizers, so if you were already planning on fertilizing your plants, you may want to save yourself the trouble of finding a separate soil additive by simply picking a fertilizer containing this type of urea.
- Sulfur-coated urea content will vary from fertilizer to fertilizer, so consult the instructions that come with your fertilizer to determine the right amount to use for your gardening needs.
- Sulfur-coated urea is a slow-release fertilizer that doses nutrients out slowly as your plants need them, rather than distributing them all at once.
-
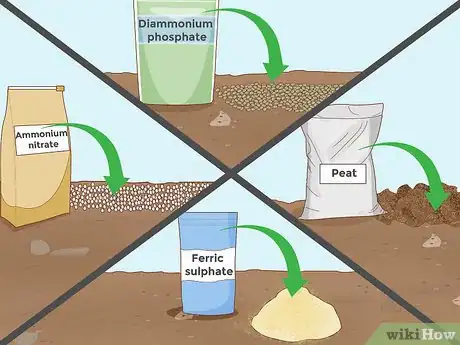
5Add another acidic additive. Besides the additives listed above, many other substances can lower soil pH. Many of these substances are often included in certain fertilizer mixes, while others are sold on their own. The time and amount needed can vary greatly for each, so consult the additive packaging or talk to an experienced employee at the gardening supply store. Additives that can lower your soil pH:[9]
- Diammonium phosphate
- Ferric sulfate
- Peat
- Ammonium nitrate
-
6Grow alkaline-tolerant plants. If your soil is too alkaline (basic) to grow plants which require acidic soil, raising alkaline-loving plants can gradually lower the pH of your soil over the life of the plant. As the plant grows, matures, and decays, the organic matter that returns to the soil will promote the growth of bacteria and gradually lower the pH level of the soil (similar to how adding organic matter in the form of mulch or manure). This method is generally one of the slowest ways to lower your soil pH, as the plant must grow to begin depositing organic matter in the soil. A few examples of alkaline-tolerant plants:[10]
- Certain evergreen shrubs (e.g., boxwoods, California lilacs)
- Certain deciduous shrubs (e.g., lilacs, mock oranges, Forsythia species)
- Certain perennials (e.g., pinks, hellebores)
Identifying Plants That Prefer a Lower Soil pH
-
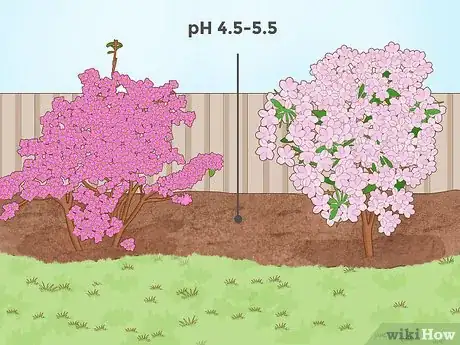
1Lower soil pH for shrubs like rhododendrons and azaleas. Certain types of flowering shrubs, like rhododendron and azalea plants, require fairly acidic soil to grow properly. These plants are often native to areas with a high amount of rainfall, like the Pacific Northwest region of the United States (frequent rainfall generally lowers soil pH).[11] For these types of shrubs, a pH range of about 4.5 - 5.5 is optimal. However, pH levels as high as 6.0 are usually acceptable.[12]
-
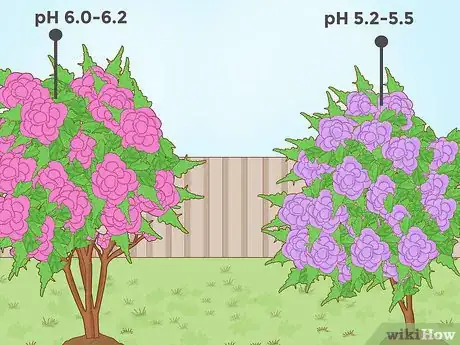
2Lower soil pH for flowers like begonias and hydrangeas. Many brightly-colored flowers, like petunias and begonias, grow best in acidic soils. For some of these flowers, changing a soil acidity from slightly acidic to very acidic can produce a visible change in the bloom color. For instance, growing a hydrangea in soil with a pH level of about 6.0 - 6.2 will produce a plant with pink flowers, while lowering the pH level to about 5.2 - 5.5 will produce a plant with purple/blue flowers.[13]
- The blue color of low-pH hydrangeas comes from the chemical aluminum. When the pH of the soil is low, it's easier for the hydrangea to absorb aluminum from the soil, which shows in the flower petals.[14]
-
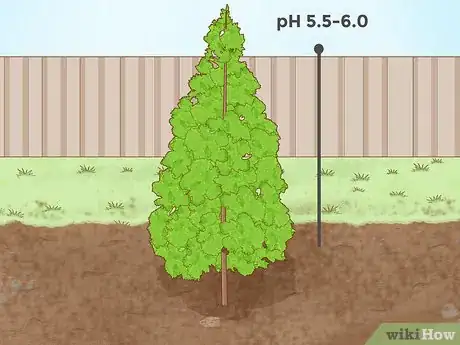
3Lower soil pH for evergreen trees. Many needled evergreen trees grow in slightly acidic soil. For instance, spruces, firs, and pines all thrive in soils with a pH level of about 5.5 - 6.0. In addition, the needles of these types of trees can be introduced to neutral or alkaline soil as organic matter which lowers soil pH as the needles decay.
-
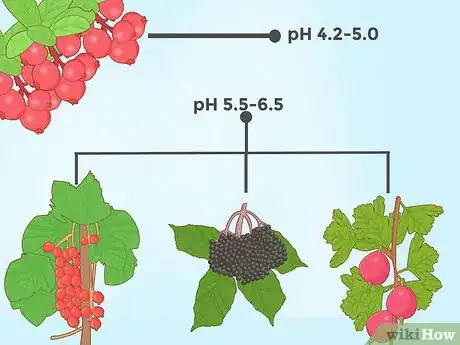
4Reduce the soil pH for certain berries. Perhaps the most well-known acid-loving plant is the blueberry, which thrives in highly acidic soils (generally about 4.0 - 5.0 is ideal). However, several other species of berry also favor acidic soil. For instance, cranberries grow best at a level of about 4.2 - 5.0, while gooseberries, currants, and elderberries grow best at a level of about 5.5 - 6.5.[15]
-
5Drop the pH to just below neutral for ferns. Most varieties of garden ferns prefer soil pH levels below 7.0 — even those that prefer alkaline soils can usually tolerate slightly acidic soils. For instance, Maidenhair Ferns prefer soil pHs of about 7.0 - 8.0 but can grow even in soils with levels of about 6.0.[16] Some ferns can even tolerate soils with a pH as low as 4.0.[17]
-
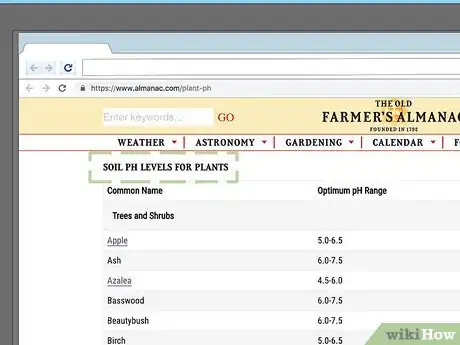
6Consult a gardening resource for a comprehensive list of acid-loving plants. The number of plants that survive or thrive in low pH soils is too great to list in this article. For more information, you may want to consult a comprehensive botanical resource. You can usually find these at gardening stores or specialty book stores, though many are also available. For instance, the official site of The Old Farmer's Almanac contains a table listing the pH preferences of many different types of plants (you can access it here).
Expert Q&A
Did you know you can get premium answers for this article?
Unlock premium answers by supporting wikiHow
-
QuestionWill magnesium sulfate lower soil pH?
 Andrew Carberry, MPHAndrew Carberry is a Food Systems Expert and the Senior Program Associate at the Wallace Centere at Winrock International in Little Rock, Arkansas. He has worked in food systems since 2008 and has experience working on farm-to-school projects, food safety programs, and working with local and state coalitions in Arkansas. He is a graduate of the College of William and Mary and holds a Masters degree in public health and nutrition from the University of Tennessee.
Andrew Carberry, MPHAndrew Carberry is a Food Systems Expert and the Senior Program Associate at the Wallace Centere at Winrock International in Little Rock, Arkansas. He has worked in food systems since 2008 and has experience working on farm-to-school projects, food safety programs, and working with local and state coalitions in Arkansas. He is a graduate of the College of William and Mary and holds a Masters degree in public health and nutrition from the University of Tennessee.
Food Systems Expert
-
QuestionHow can a farmer convert acidic soil to neutral soil?
 Andrew Carberry, MPHAndrew Carberry is a Food Systems Expert and the Senior Program Associate at the Wallace Centere at Winrock International in Little Rock, Arkansas. He has worked in food systems since 2008 and has experience working on farm-to-school projects, food safety programs, and working with local and state coalitions in Arkansas. He is a graduate of the College of William and Mary and holds a Masters degree in public health and nutrition from the University of Tennessee.
Andrew Carberry, MPHAndrew Carberry is a Food Systems Expert and the Senior Program Associate at the Wallace Centere at Winrock International in Little Rock, Arkansas. He has worked in food systems since 2008 and has experience working on farm-to-school projects, food safety programs, and working with local and state coalitions in Arkansas. He is a graduate of the College of William and Mary and holds a Masters degree in public health and nutrition from the University of Tennessee.
Food Systems Expert
-
QuestionHow can you acidify the soil?
 wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
Staff Answer wikiHow Staff EditorStaff AnswerTry testing the soil first, so you can be sure how much acidification is needed. You’ll also need to identify the soil type. After doing that, you can consider adding things that are suited to the soil type and amount of acidification needed, such as elemental sulfur, iron sulfate or fertilizer containing ammonia. For more help with acidifying soil safely, see the wikiHow How to Acidify Soil.
wikiHow Staff EditorStaff AnswerTry testing the soil first, so you can be sure how much acidification is needed. You’ll also need to identify the soil type. After doing that, you can consider adding things that are suited to the soil type and amount of acidification needed, such as elemental sulfur, iron sulfate or fertilizer containing ammonia. For more help with acidifying soil safely, see the wikiHow How to Acidify Soil.
Warnings
- Too much aluminum sulfate can poison the soil.⧼thumbs_response⧽
- If you spill urea, aluminum sulfate, or sulfur on the leaves of your plant, rinse it off with plenty of clear water. Allowing it to sit on the plant leaves can "burn" them, causing unsightly damage.⧼thumbs_response⧽
References
- ↑ https://www.extension.purdue.edu/extmedia/HO/HO-241-W.pdf
- ↑ http://www.thegardenhelper.com/acidsoil.html
- ↑ http://www.dummies.com/how-to/content/how-to-improve-garden-soil-with-organic-matter.html
- ↑ http://www.clemson.edu/extension/hgic/plants/other/soils/hgic1650.html
- ↑ http://www.clemson.edu/extension/hgic/plants/other/soils/hgic1650.html
- ↑ http://www.clemson.edu/extension/hgic/plants/other/soils/hgic1650.html
- ↑ http://www.clemson.edu/extension/hgic/plants/other/soils/hgic1650.html
- ↑ http://www.extension.umn.edu/agriculture/nutrient-management/nitrogen/fertilizer-urea/
- ↑ https://www.extension.purdue.edu/extmedia/HO/HO-241-W.pdf
- ↑ http://www.hortmag.com/headline/plants-for-alkaline-soil
- ↑ http://www.rhododendron.org/v46n2p77.htm
- ↑ http://www.rhododendron.org/v46n2p77.htm
- ↑ http://www.hydrangeashydrangeas.com/colorchange.html
- ↑ http://www.hydrangeashydrangeas.com/colorchange.html
- ↑ http://www.offthegridnews.com/2013/06/19/25-fruits-and-vegetables-to-grow-in-acidic-soil/
- ↑ http://www.whiteflowerfarm.com/growing-and-planting-garden-ferns.html
- ↑ http://www.whiteflowerfarm.com/growing-and-planting-garden-ferns.html
- http://www.almanac.com/content/ph-preferences
- http://www.savvygardener.com/Features/soil_ph.html
- http://compostguide.com/making-compost-the-basics/
About This Article
To lower soil pH, mix some aluminum sulfate into the soil, which will instantly lower the pH level. For a cheaper option that takes slightly longer, add some sublimed sulfur to the soil. If you're not in a rush to lower the soil's pH, mix in some organic matter, like compost, composted manure, or acidic mulch, which will gradually lower the pH level. To learn how to perform a pH test, keep reading!