The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units (SI) and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using absolute zero as its null point. In the classical description of thermodynamics, absolute zero is the temperature at which all thermal motion ceases.
The choice of absolute zero as null point for the Kelvin scale is logical. Different types of matter boil or freeze at different temperatures, but at 0K (absolute zero), all thermal motions of any matter are maximally suppressed. The Kelvin scale is used extensively in scientific work because a number of physical quantities, such as the volume of an ideal gas, are directly related to absolute temperature.
The Kelvin scale is named after Glasgow University engineer and physicist William Thomson, 1st Baron Kelvin (1824-1907), who wrote of the need for an "absolute thermometric scale. " Unlike the degree Fahrenheit and the degree Celsius, the kelvin is not referred to or typeset as a degree. The kelvin is the primary unit of measurement in the physical sciences, but it is often used in conjunction with the degree Celsius, which has the same magnitude. The kelvin is defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water (exactly 0.01°C, or 32.018°F). To convert kelvin to degrees Celsius, we use the following formula:
Subtracting 273.16K from the temperature of the triple point of water, 0.01°C, makes absolute zero (0K) equivalent to -273.15°C and -460°F .
Calculating U
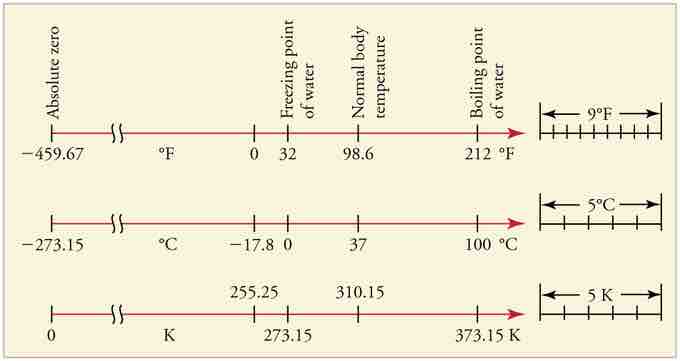
Relationships Between the Temperature Scales
Relationships between the Fahrenheit, Celsius, and Kelvin temperature scales, rounded to the nearest degree. The relative sizes of the scales are also shown