The uncertainty principle is any of a variety of mathematical inequalities, asserting a fundamental limit to the precision with which certain pairs of physical properties of a particle, such as position x and momentum p or energy E and time t, can be known simultaneously. The more precisely the position of some particle is determined, the less precisely its momentum can be known, and vice versa. This can be formulated as the following inequality:
The principle is quite counterintuitive, so the early students of quantum theory had to be reassured that naive measurements to violate it were bound always to be unworkable. One way in which Heisenberg originally illustrated the intrinsic impossibility of violating the uncertainty principle is by using an imaginary microscope (see ) as a measuring device.
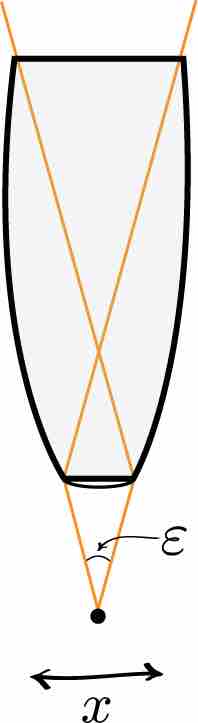
Heisenberg Microscope
Heisenberg's microscope, with cone of light rays focusing on a particle with angle \epsilon
He imagines an experimenter trying to measure the position and momentum of an electron by shooting a photon at it.
Example One
If the photon has a short wavelength and therefore a large momentum, the position can be measured accurately. But the photon scatters in a random direction, transferring a large and uncertain amount of momentum to the electron. If the photon has a long wavelength and low momentum, the collision does not disturb the electron's momentum very much, but the scattering will reveal its position only vaguely.
Example Two
If a large aperture is used for the microscope, the electron's location can be well resolved (see Rayleigh criterion); but by the principle of conservation of momentum, the transverse momentum of the incoming photon and hence the new momentum of the electron resolves poorly. If a small aperture is used, the accuracy of both resolutions is the other way around.
Heisenberg's Argument
Heisenberg's argument is summarized as follows. He begins by supposing that an electron is like a classical particle, moving in the x direction along a line below the microscope, as in the illustration to the right. Let the cone of light rays leaving the microscope lens and focusing on the electron makes an angle
It is at this point that Heisenberg introduces objective indeterminacy into the thought experiment. He writes that "the recoil cannot be exactly known, since the direction of the scattered photon is undetermined within the bundle of rays entering the microscope". In particular, the electron's momentum in the x direction is only determined up to