Concept
Version 11
Created by Boundless
Zero-Order Reactions
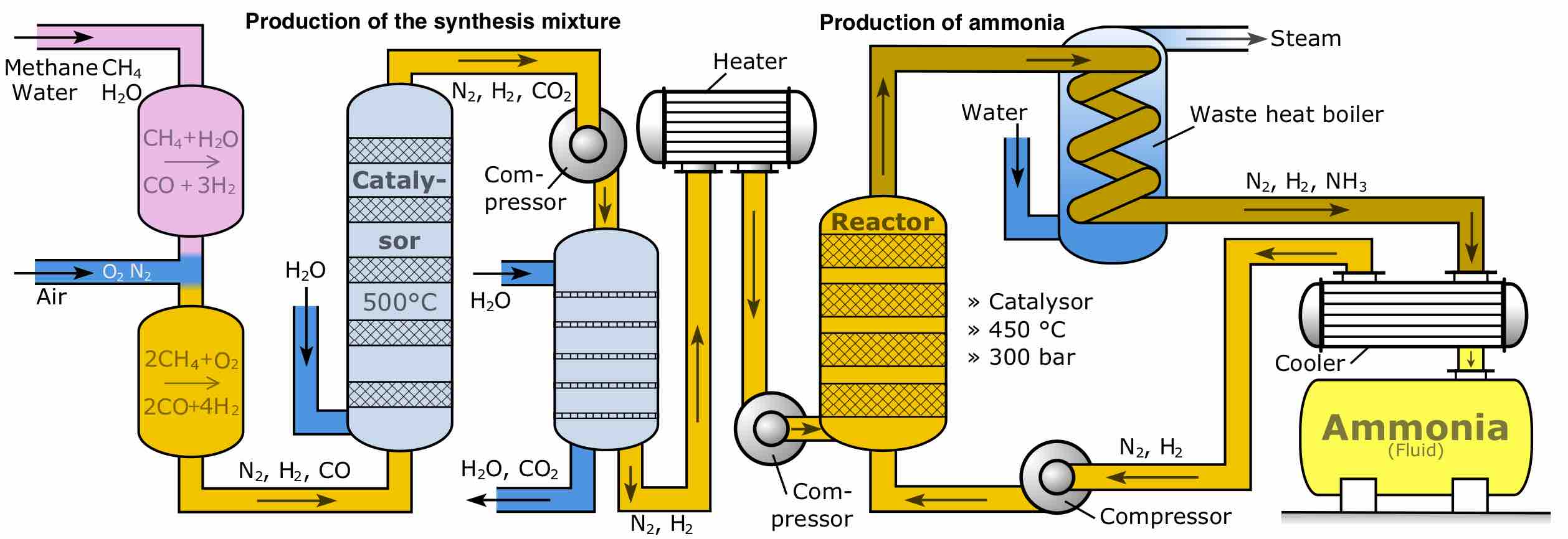
The Haber process
The Haber process produces ammonia from hydrogen and nitrogen gas. The reverse of this process (the decomposition of ammonia to form nitrogen and hydrogen) is a zero-order reaction.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"File:Haber-Bosch-En.svg - Wikimedia Commons."
http://commons.wikimedia.org/w/index.php?title=File:Haber-Bosch-En.svg&page=1
Wikimedia
CC BY-SA.