Reaction Mechanisms
Every chemical reaction proceeds according to a reaction mechanism, which is a step-by-step description of what occurs during a reaction on the molecular level. Each step of the mechanism is known as an elementary process, which describes a single moment during a reaction in which molecules break and/or form new bonds. It is important to keep in mind that every reaction mechanism is simply a proposed version of what might be occurring at the molecular level; even if a mechanism agrees with experimental results, it is impossible to prove a reaction mechanism for certain. That being said, there are two strict requirements that must be fulfilled for a reaction mechanism to be valid. They are:
- The sum of each elementary step in a reaction mechanism must yield the overall reaction equation.
- The rate law of the rate-determining step must agree with the experimentally determined rate law.
The rate-determining step is the slowest step in a reaction mechanism. Because it is the slowest, it determines the rate of the overall reaction. This will be explored later in more detail.
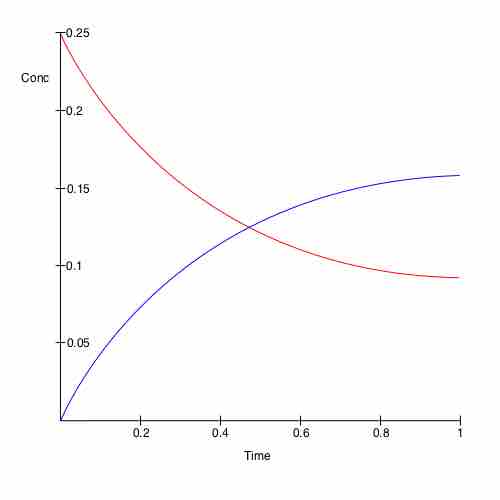
Change in concentration of chemicals over time
A plot of time versus concentration for two species in chemical equilibrium.
Elementary Reactions and Their Rate Laws
Elementary reactions are classified in terms of their molecularity; that is, by how many molecules are involved in the given step. There are three possible molecularites:
-
unimolecular (
$A \rightarrow products$ ) -
bimolecular (
$A + B \rightarrow products$ ) - termolecular (
$A + B + C \rightarrow products$ )
Termolecularity is not common due to the rarity of three molecules colliding at the same time, and in just the right way for reaction to occur.
The molecularity of the elementary step, and the reactants involved, will determine what the rate law will be for that particular step in the mechanism.
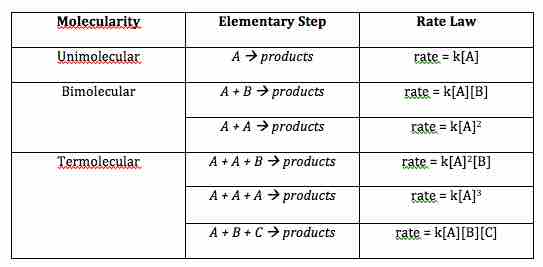
Molecularity of elementary steps and corresponding rate laws
The molecularity of an elementary step in a reaction mechanism determines the form of its rate law.
Example of Elementary Steps and Their Rate Laws
Consider the following reaction:
The overall equation suggests that two NO molecules collide with an oxygen molecule, forming NO2. However, because termolecular reactions are extremely rare, this reaction most likely consists of two or more elementary steps. Upon further analysis, the mechanism is given as:
Note that the two steps here add to the overall reaction equation, as the intermediate N2O2 cancels. However, we cannot simply add the rate laws of each elementary step in order to get the overall reaction rate. Determining the overall reaction rate from the reaction mechanism will be discussed in the next concept. For now, just keep in mind that the rate laws for each elementary step are determined by the molecularity of each step only.