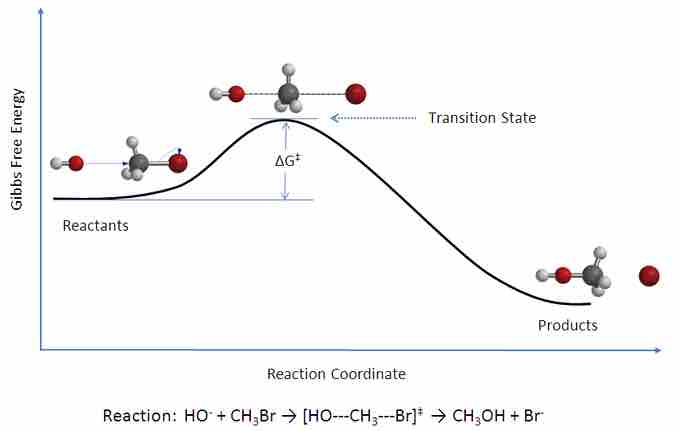
Transition state theory (TST) describes a hypothetical "transition state" that occurs in the space between the reactants and the products in a chemical reaction. The species that is formed during the transition state is known as the activated complex. TST is used to describe how a chemical reaction occurs, and it is based upon collision theory. If the rate constant for a reaction is known, TST can be used successfully to calculate the standard enthalpy of activation, the standard entropy of activation, and the standard Gibbs energy of activation. TST is also referred to as "activated-complex theory," "absolute-rate theory," and "theory of absolute reaction rates."
Transition state theory
The activated complex, which a kind of reactant-product hybrid, exists at the peak of the reaction coordinate, in what is known as the transition state.
Postulates of Transition State Theory
According to transition state theory, between the state in which molecules exist as reactants and the state in which they exist as products, there is an intermediate state known as the transition state. The species that forms during the transition state is a higher-energy species known as the activated complex. TST postulates three major factors that determine whether or not a reaction will occur. These factors are:
- The concentration of the activated complex.
- The rate at which the activated complex breaks apart.
- The mechanism by which the activated complex breaks apart; it can either be converted into products, or it can "revert" back to reactants.
This third postulate acts as a kind of qualifier for something we have already explored in our discussion on collision theory. According to collision theory, a successful collision is one in which molecules collide with enough energy and with proper orientation, so that reaction will occur. However, according to transition state theory, a successful collision will not necessarily lead to product formation, but only to the formation of the activated complex. Once the activated complex is formed, it can then continue its transformation into products, or it can revert back to reactants.
Applications in Biochemistry
Transition state theory is most useful in the field of biochemistry, where it is often used to model reactions catalyzed by enzymes in the body. For instance, by knowing the possible transition states that can form in a given reaction, as well as knowing the various activation energies for each transition state, it becomes possible to predict the course of a biochemical reaction, and to determine its reaction rate and rate constant.