Section 4
Reaction Mechanisms
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
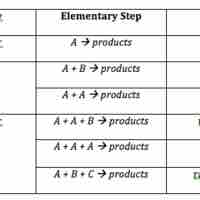
Rate Laws for Elementary Steps
The rate law for an elementary step is derived from the molecularity of that step.

Rate-Determining Steps
The rate of a multi-step reaction is determined by the slowest elementary step, which is known as the rate-determining step.
Overall Reaction Rate Laws
Rate laws for reactions are affected by the position of the rate-determining step in the overall reaction mechanism.
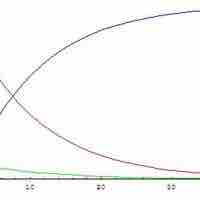
Steady-State Approximation
The steady state approximation can be used to determine the overall rate law when the rate-determining step is unknown.
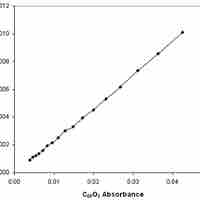
Experimental Determination of Reaction Rates
Reaction rates can be determined experimentally by measuring the concentration of a reactant and/or product over time.