Deviations from the Octet Rule
A hypervalent molecule is a molecule that contains one or more main group elements that bear more than eight electrons in their valence levels as a result of bonding. Phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), chlorine trifluoride (ClF3), and the triiodide ion (I3−) are examples of hypervalent molecules.
For the elements in the second period of the periodic table (principal energy level n=2), the s2p6 electrons comprise the octet, and no d sublevel exists. As a result, the second period elements (more specifically, the nonmetals C, N, O, F) obey the octet rule without exceptions.
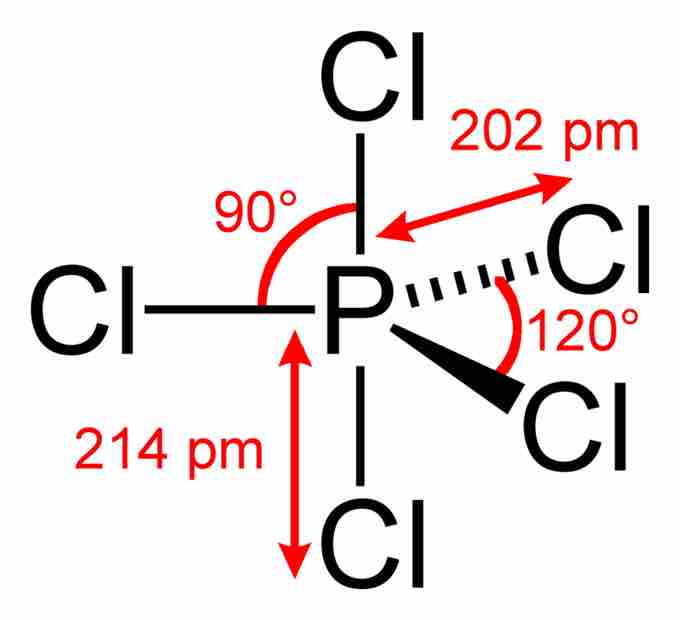
Phosphorus pentachloride
In the PCl5 molecule, the central phosphorus atom is bonded to five Cl atoms, thus having 10 bonding electrons and violating the octet rule. The overall geometry of the molecule is depicted (trigonal bipyramidal), and bond angles and lengths are highlighted.
However, some of the third-period elements (Si, P, S, and Cl) have been observed to bond to more than four other atoms, and thus need to involve more than the four pairs of electrons available in an s2p6 octet. This is possible because for n=3, the d sublevel exists, and it has five d orbitals. Although the energy of empty 3d-orbitals is ordinarily higher than that of the 4s orbital, that difference is small and the additional d orbitals can accommodate more electrons. Therefore, the d orbitals participate in bonding with other atoms and an expanded octet is produced. Examples of molecules in which a third period central atom contains an expanded octet are the phosphorus pentahalides and sulfur hexafluoride.
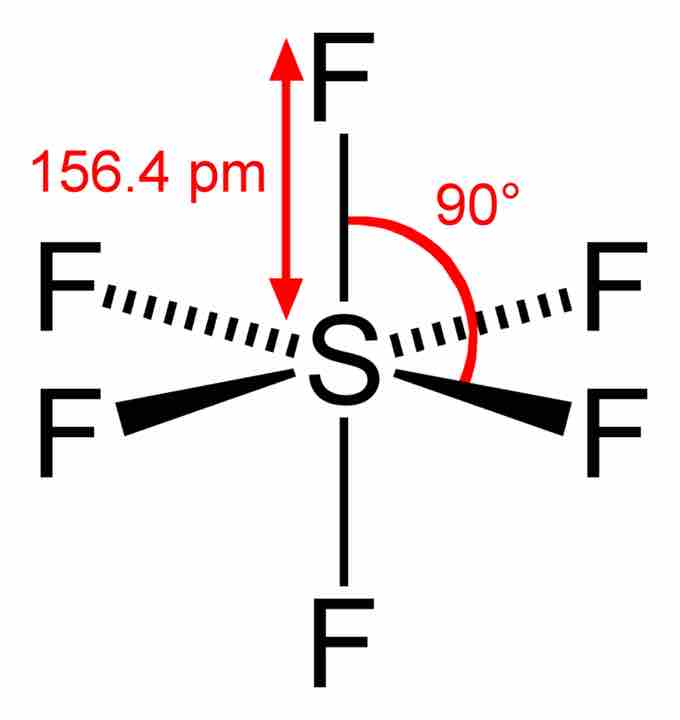
Sulfur hexafluoride
In the SF6 molecule, the central sulfur atom is bonded to six fluorine atoms, so sulfur has 12 bonding electrons around it. The overall geometry of the molecule is depicted (tetragonal bipyramidal, or octahedral), and bond angles and lengths are highlighted.
For atoms in the fourth period and beyond, higher d orbitals can be used to accommodate additional shared pairs beyond the octet. The relative energies of the different kinds of atomic orbital reveal that energy gaps become smaller as the principal energy level quantum number (n) increases, and the energetic cost of using these higher orbitals to accommodate bonding electrons becomes smaller.