Concept
Version 10
Created by Boundless
The Expanded Octet
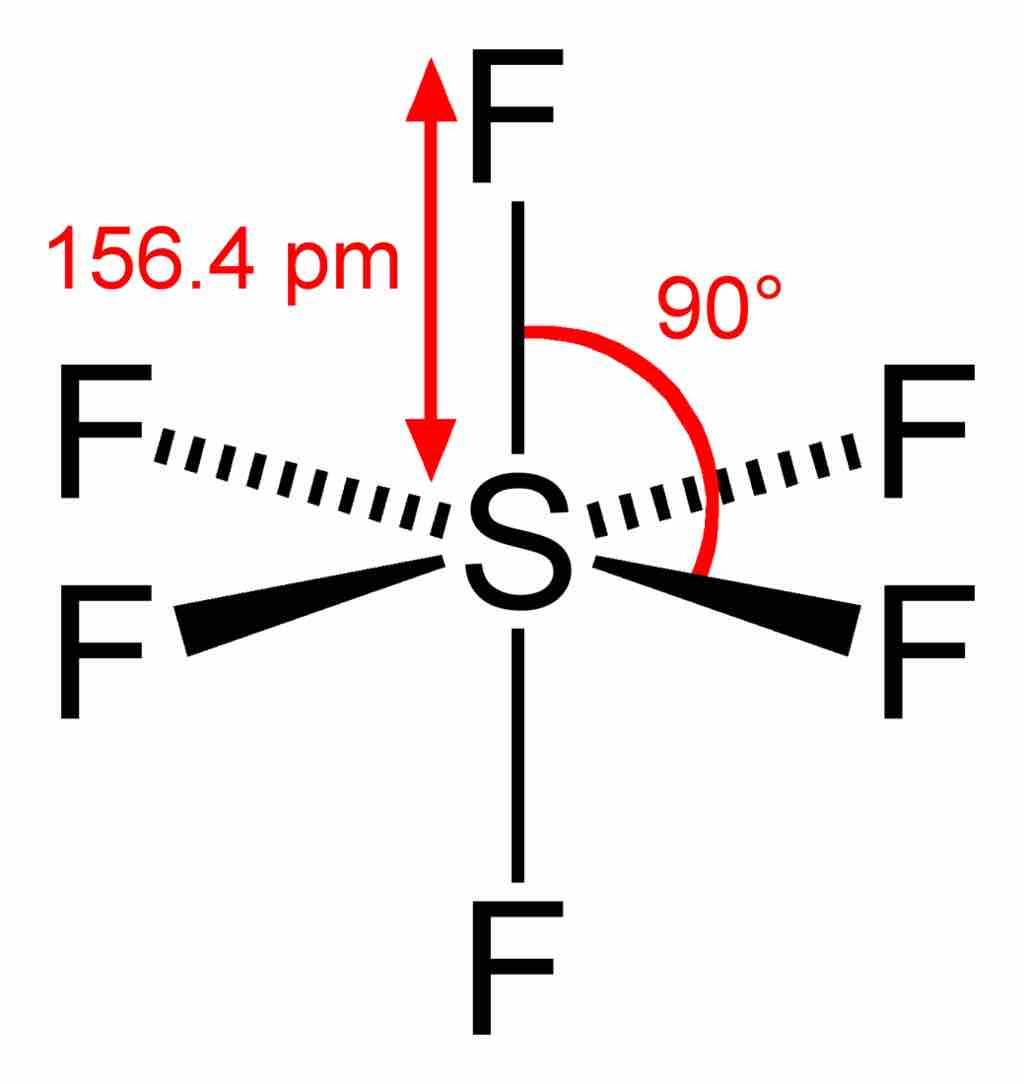
Sulfur hexafluoride
In the SF6 molecule, the central sulfur atom is bonded to six fluorine atoms, so sulfur has 12 bonding electrons around it. The overall geometry of the molecule is depicted (tetragonal bipyramidal, or octahedral), and bond angles and lengths are highlighted.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Sulfur-hexafluoride-2D-dimensions."
http://en.wikipedia.org/wiki/File:Sulfur-hexafluoride-2D-dimensions.png
Wikipedia
CC BY-SA.