Section 2
Buffer Solutions
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts

Preparing a Buffer Solution with a Specific pH
A buffer is a solution of weak acid and conjugate base or weak base and conjugate acid used to resist pH change with added solute.
Calculating the pH of a Buffer Solution
The pH of a buffer solution can be calculated from the equilibrium constant and the initial concentration of the acid.
The Henderson-Hasselbalch Equation
The Henderson–Hasselbalch equation connects the measurable value of the pH of a solution with the theoretical value pKa.
Calculating Changes in a Buffer Solution
The changed pH of a buffer solution in response to the addition of an acid or a base can be calculated.
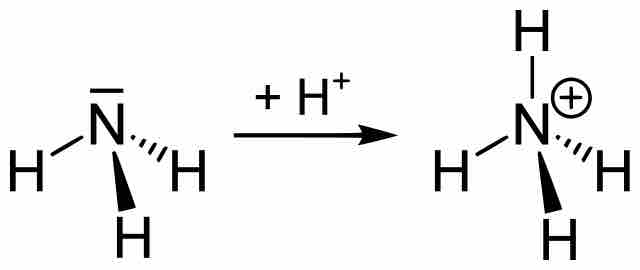
Buffers Containing a Base and Conjugate Acid
An alkaline buffer can be made from a mixture of the base and its conjugate acid, but the formulas for determining pH take a different form.