X
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 11 people, some anonymous, worked to edit and improve it over time.
This article has been viewed 24,716 times.
Learn more...
Glow sticks are a type of light source that contains two chemicals and a dye called sensitizer or fluorophosphate. [1] This creates an endothermic reaction and causes the glow sticks to glow. If the glow sticks are too dim for you liking, you can brighten them up by speeding up the heat and reaction.
Steps
Method 1
Method 1 of 2:
Boiling Water Method
-
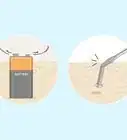
1Snap the glow sticks so you can activate the chemical reaction needed for the sticks to glow.
-
2Fill a pan with warm water and have it boil.Advertisement
-
3Place the glow sticks in the water and allow it to boil for about a minute. The heat will speed up the chemical reaction, making the glow sticks twice as brighter than they were originally. [2]
-
4Remove the glow sticks from the water using tongs.
-
5Go in a dark room. Now your glow stick can glow brighter!
Advertisement
Method 2
Method 2 of 2:
Microwave Method
-
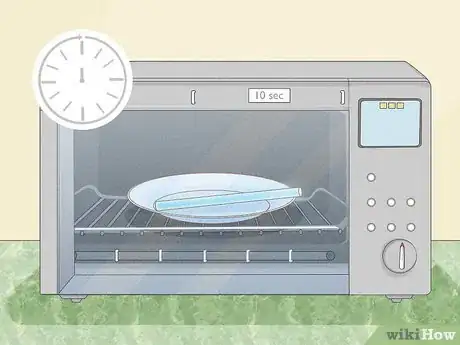
1Snap the glow sticks so you can activate the chemical reaction needed for the sticks to glow.
-
2Place them on a plate.
-
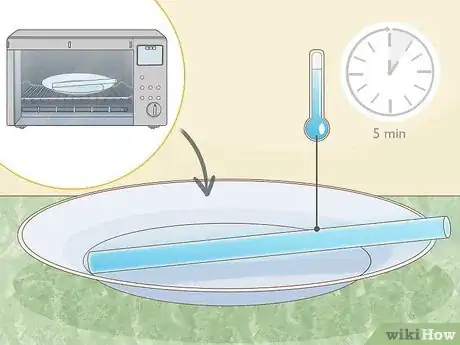
3Microwave them on high for about ten seconds. The heat from the microwave will energize the hydrogen peroxide in the glow sticks, making the glow sticks react faster and brighter. .[3]
-
4Remove the glow sticks from the microwave and allow them to cool for about five minutes.
-
5Go in a dark room. Now your glow stick can glow brighter!
Advertisement
Community Q&A
-
QuestionHow does this work chemically?
 TomPNTop AnswererThe heat/microwaves add energy to the reaction, causing it to proceed faster and release more photons per second, which we perceive as an increase in brightness.
TomPNTop AnswererThe heat/microwaves add energy to the reaction, causing it to proceed faster and release more photons per second, which we perceive as an increase in brightness. -
QuestionHow do I make a glow stick?
 Anika ShenoyCommunity AnswerLook at our article How to Make a Glowstick. This shows you how to create a Glowstick in just a few steps!
Anika ShenoyCommunity AnswerLook at our article How to Make a Glowstick. This shows you how to create a Glowstick in just a few steps! -
QuestionWhat are important facts about glow sticks?
 TomPNTop AnswererThe contain hydrogen peroxide, a diphenyl oxalate derivative (usually bis( 2,4,6-trichlorophenyl) oxalate), and a fluorophore dye. There is a glass capsule in the center, which separates the peroxide and oxalate chemicals. When it is broken, the chemicals react together, producing energy that is captured by the dye and emitted as light. The type of dye determines the color.
TomPNTop AnswererThe contain hydrogen peroxide, a diphenyl oxalate derivative (usually bis( 2,4,6-trichlorophenyl) oxalate), and a fluorophore dye. There is a glass capsule in the center, which separates the peroxide and oxalate chemicals. When it is broken, the chemicals react together, producing energy that is captured by the dye and emitted as light. The type of dye determines the color.
Advertisement
Warnings
- Take caution not to over-heat the glow sticks or they will pop. [5]⧼thumbs_response⧽
Advertisement
References
- ↑ https://en.wikipedia.org/wiki/Glow_stick
- ↑ http://www.ehow.com/how_4843284_glow-sticks-brighter.html
- ↑ http://www.instructables.com/id/Make-your-glow-sticks-SUPER-Bright/
- ↑ http://www.instructables.com/id/Make-your-glow-sticks-SUPER-Bright/
- ↑ http://www.instructables.com/id/Make-your-glow-sticks-SUPER-Bright/
About This Article
Advertisement