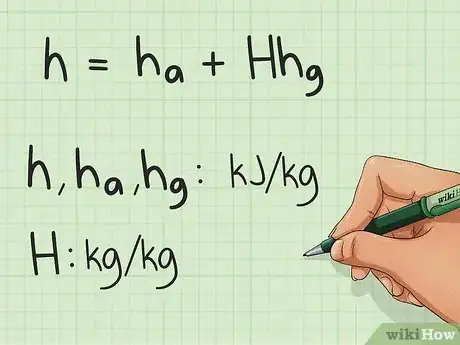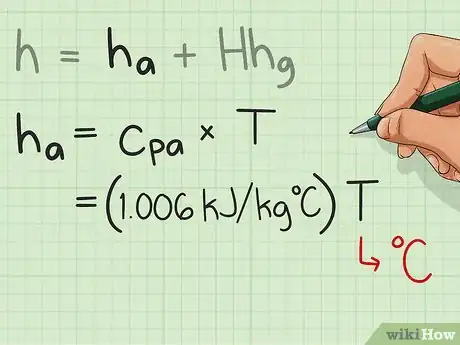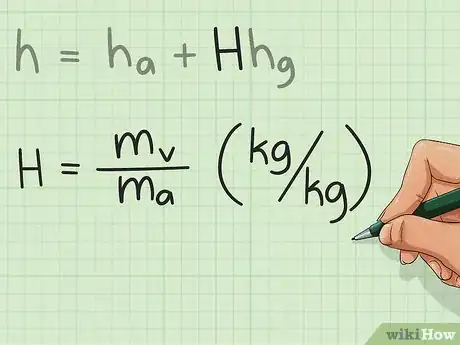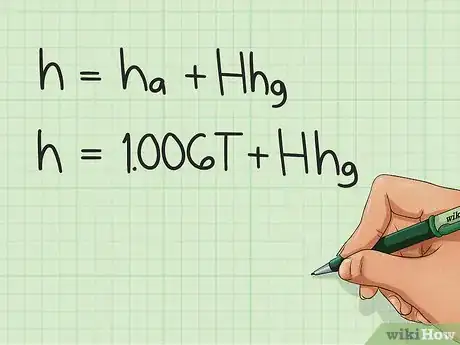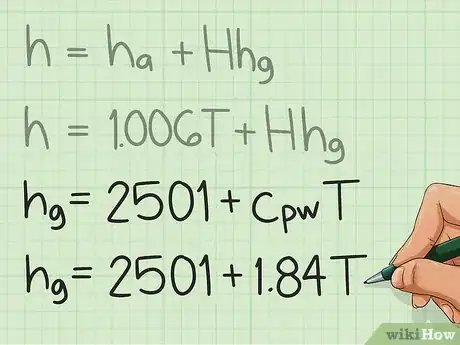A step-by-step guide on air enthalpy calculation (what formula to use and more)
X
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 9 people, some anonymous, worked to edit and improve it over time.
This article has been viewed 52,542 times.
Learn more...
Psychrometry is the study of properties of air, which is an important part of the field of Air-conditioning. One important property is the enthalpy, which is a measure of the energy present in the substance at a certain state.
Things You Should Know
- The equation for enthalpy is h = ha + H*hg where ha is the specific enthalpy of dry air, H is the humidity ratio, and hg is the specific enthalpy of water vapor.
- You can look online for a steam table and use that to determine hg (the specific enthalpy of water vapor).
- If you have to calculate the specific enthalpy of water vapor manually without a steam table, use the formula 2501 kJ/kg + cpw*T.
Steps
Method 1
Method 1 of 2:
With Steam Tables
-
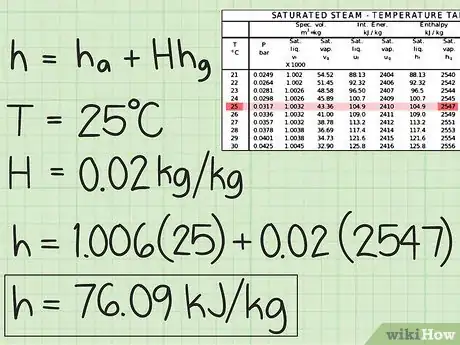
1Study the equation for calculating the enthalpy. Specific enthalpy (enthalpy per kg of dry air) of moist air is the sum of the specific enthalpy of dry air and the specific enthalpy of the water vapor in the air. This is given by the equation: h = ha + H*hg; where ha is the specific enthalpy of dry air, H is the humidity ratio, and hg is the specific enthalpy of water vapor.
-
2Calculate ha. This is given by the equation cpa*T; where cpa is the specific heat of air, and T is the temperature of air.
- You have to memorize the value of cpa which is 1.006 kJ/kgC since this is constant. T must be given in the problem, or it can simply be measured with a thermometer or other temperature measuring devices.
Advertisement -
3Examine H. Humidity ratio is the ratio of the mass of water vapor in the moist air and the mass of dry air. In layman's terms, it gives the wetness of air. Along with the temperature of air, this must be given in the problem.
-
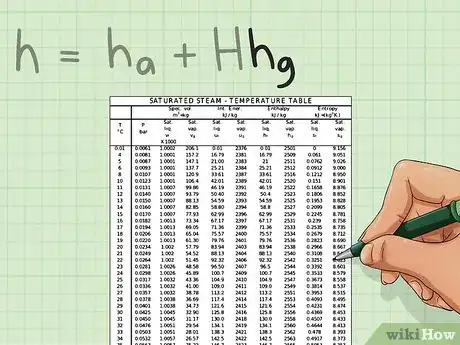
4Get hg. Using a steam table, this can easily be found. Once you have the temperature, bring out your saturated steam table and look for the value of hg. This value has the units kJ/kg.
-
5Practice. Find the specific enthalpy of moist air at 25°C with 0.02 kg/kg moisture. These are the T and H, respectively.
- Get ha. Use the equation given above and the given T in the problem.
- Get hg. Use the given T and your steam table.
- Get h. Use your calculated values of ha and hg, and the given value of H. If your answer is 76.09 kJ/kg or something very close to that, you got the right answer!
Advertisement
Method 2
Method 2 of 2:
Without Steam Tables
-
1Derive the equation. Start with the original equation h = ha + Hhg.
-
2Expand hg. If you have no access to a steam table, hg can be expressed as: hg = 2501 kJ/kg + cpw*T.
- You must memorize the value 2501 kJ/kg, which is the specific enthalpy of water vapor at 0°C.
- Therefore, to get the specific enthalpy at any temperature, you must add the term cpw*T; where cpw = 1.84 kJ/kg°C, which is the specific heat of water vapor at constant pressure; and T is the temperature of air.
-
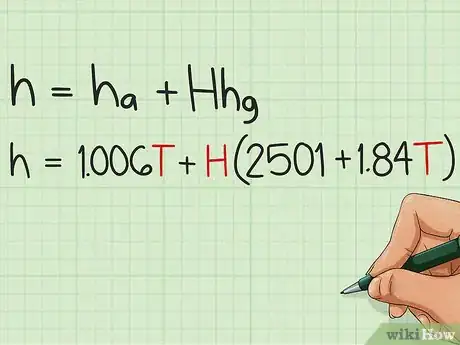
3Write the final equation. h = cpa*T + H*(2501 kJ/kg + cpw*T). As you can see, you only need T and H from the problem, and memorize the values of cpa, cpw and 2501 kJ/kg to solve any problem.
-
4Practice. Find the specific enthalpy of moist air at 25°C with 0.02 kg/kg moisture. If your answer is 76.09 kJ/kg or something very close to that, you got the right answer!
Advertisement
Community Q&A
-
QuestionHow did you get H=0.02 kg/kg?
 HanCommunity AnswerH=0.02 kg/kg is part of the given data for the specific example problem in the article.
HanCommunity AnswerH=0.02 kg/kg is part of the given data for the specific example problem in the article.
Advertisement
About This Article
Advertisement