Special Properties of Water
In general, objects will expand with increasing temperature. However, a number of materials contract on heating within certain temperature ranges; this is usually called negative thermal expansion, rather than "thermal contraction. " Water is the most important exception to the general rule. Water has this unique characteristic because of the particular nature of the hydrogen bond in H2O.
Density of Water as Temperature Changes
At temperatures greater than 4ºC (40ºF) water expands with increasing temperature (its density decreases). However, it expands with decreasing temperature when it is between +4ºC and 0ºC (40ºF to 32ºF). Water is densest at +4ºC .
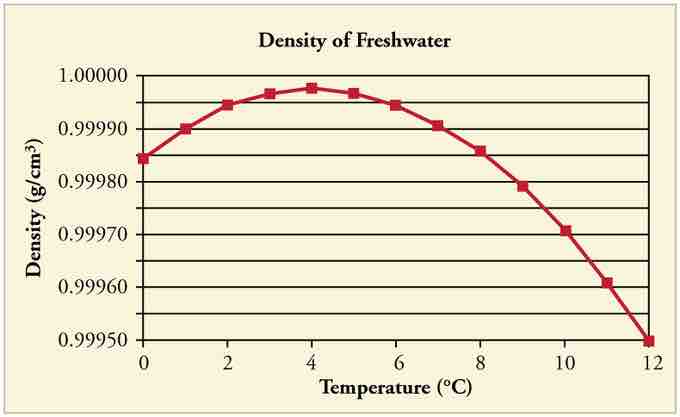
Water Density vs. Temperature
The density of water as a function of temperature. Note that the thermal expansion is actually very small. The maximum density at +4ºC is only 0.0075% greater than the density at 2ºC, and 0.012% greater than that at 0ºC.
Perhaps the most striking effect of this phenomenon is the freezing of water in a pond. When water near the surface cools down to 4ºC it is denser than the remaining water and thus will sink to the bottom. This "turnover" results in a layer of warmer water near the surface, which is then cooled. Eventually the pond has a uniform temperature of 4ºC. If the temperature in the surface layer drops below 4ºC, the water is less dense than the water below, and thus stays near the top.
As a result, the pond surface can completely freeze over, while the bottom may remain at 4ºC . The ice on top of liquid water provides an insulating layer from winter's harsh exterior air temperatures. Fish and other aquatic life can survive in 4ºC water beneath ice, due to this unusual characteristic of water. It also produces circulation of water in the pond that is necessary for a healthy ecosystem of the body of water.
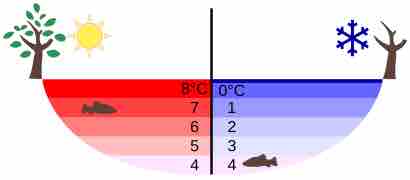
Temperature in a Lake
Temperature distribution in a lake on warm and cold days in winter
Ice Versus Water
The solid form of most substances is denser than the liquid phase; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. Upon freezing, the density of water decreases by about 9%.