Section 4
Ideal Gas Law
Book
Version 3
By Boundless
By Boundless
Boundless Physics
Physics
by Boundless
6 concepts
Equations of State
The ideal gas law is the equation of state of a hypothetical ideal gas (in which there is no molecule to molecule interaction).
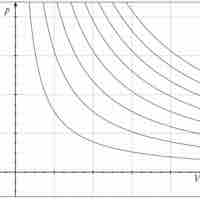
Isotherms
An isothermal process is a change of a system in which the temperature remains constant: ΔT = 0.
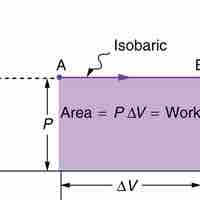
Constant Pressure
Isobaric processis a thermodynamic process in which the pressure stays constant (at constant pressure, work done by a gas is

Problem Solving
With the ideal gas law we can figure pressure, volume or temperature, and the number of moles of gases under ideal thermodynamic conditions.
Avogador's Number
The number of molecules in a mole is called Avogadro's number (NA)—defined as 6.02x 1023 mol-1.
Absolute Temperature
Absolute temperature is the most commoly used thermodyanmic temperature unit and is the standard unit of temperature.