Radiocarbon dating (usually referred to simply as carbon-14 dating) is a radiometric dating method. It uses the naturally occurring radioisotope carbon-14 (14C) to estimate the age of carbon-bearing materials up to about 58,000 to 62,000 years old.
Carbon has two stable, nonradioactive isotopes: carbon-12 (12C) and carbon-13 (13C). There are also trace amounts of the unstable radioisotope carbon-14 (14C) on Earth. Carbon-14 has a relatively short half-life of 5,730 years, meaning that the fraction of carbon-14 in a sample is halved over the course of 5,730 years due to radioactive decay to nitrogen-14. The carbon-14 isotope would vanish from Earth's atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with molecules of nitrogen (N2) and single nitrogen atoms (N) in the stratosphere. Both processes of formation and decay of carbon-14 are shown in .
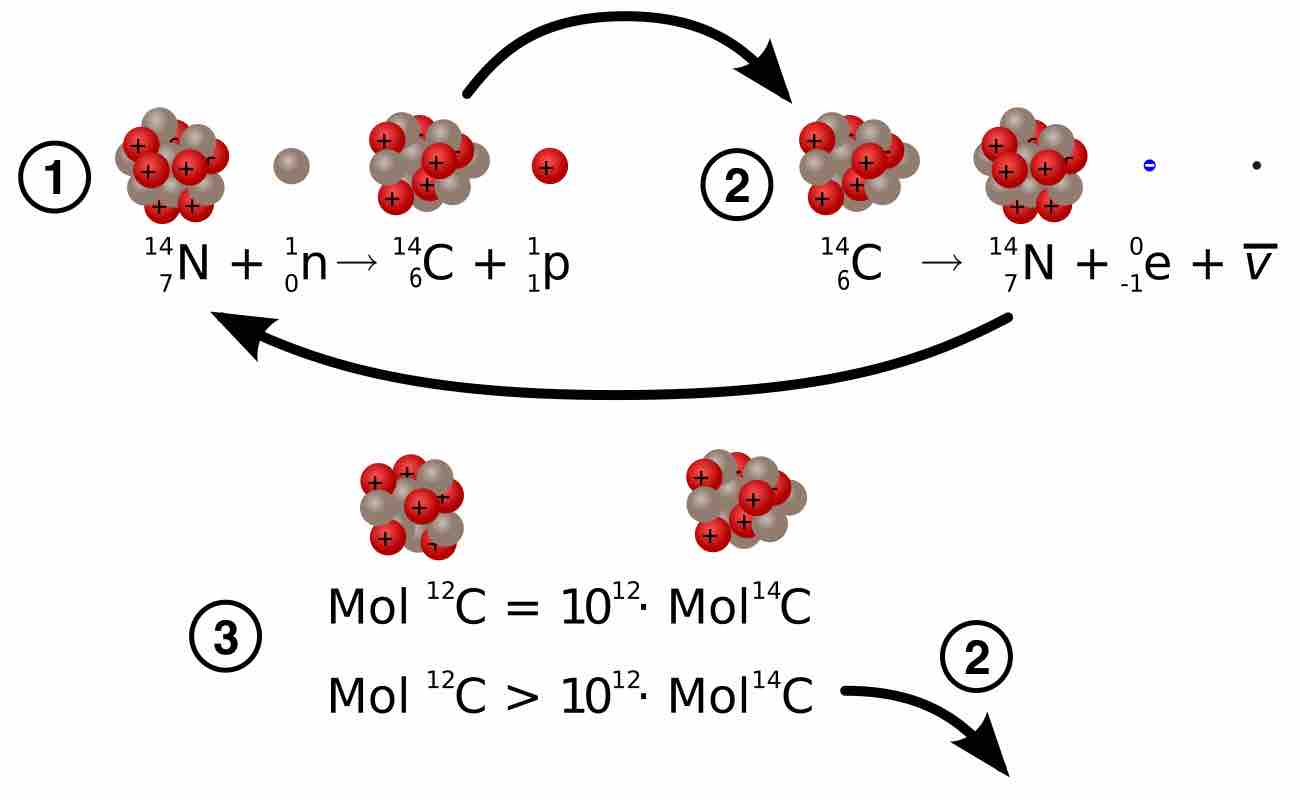
Formation and Decay of Carbon-14
Diagram of the formation of carbon-14 (1), the decay of carbon-14 (2), and equations describing the carbon-12:carbon-14 ratio in living and dead organisms
When plants fix atmospheric carbon dioxide (CO2) into organic compounds during photosynthesis, the resulting fraction of the isotope 14C in the plant tissue will match the fraction of the isotope in the atmosphere. After plants die or are consumed by other organisms, the incorporation of all carbon isotopes, including 14C, stops. Thereafter, the concentration (fraction) of 14C declines at a fixed exponential rate due to the radioactive decay of 14C. (An equation describing this process is shown in . ) Comparing the remaining 14C fraction of a sample to that expected from atmospheric 14C allows us to estimate the age of the sample.
Raw (i.e., uncalibrated) radiocarbon ages are usually reported in radiocarbon years "Before Present" (BP), with "present" defined as CE 1950. Such raw ages can be calibrated to give calendar dates. One of the most frequent uses of radiocarbon dating is to estimate the age of organic remains from archaeological sites.
The technique of radiocarbon dating was developed by Willard Libby and his colleagues at the University of Chicago in 1949. Emilio Segrè asserted in his autobiography that Enrico Fermi suggested the concept to Libby at a seminar in Chicago that year. Libby estimated that the steady-state radioactivity concentration of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram. In 1960, Libby was awarded the Nobel Prize in chemistry for this work. He demonstrated the accuracy of radiocarbon dating by accurately estimating the age of wood from a series of samples for which the age was known, including an ancient Egyptian royal barge dating from 1850 BCE.