Electrons are emitted from matter when light shines on a surface . This is called the photoelectric effect, and the electrons emitted in this manner are called photoelectrons.
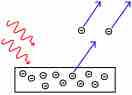
The Photoelectric Effect
Electrons are emitted from matter by absorbed light.
The photoelectric effect typically requires photons with energies from a few electronvolts to 1 MeV for heavier elements, roughly in the ultraviolet and X-ray range. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave-particle duality. The photoelectric effect is also widely used to investigate electron energy levels in matter.
Heinrich Hertz discovered the photoelectric effect in 1887. Although electrons had not been discovered yet, Hertz observed that electric currents were produced when ultraviolet light was shined on a metal. By the beginning of the 20th century, physicists confirmed that:
- The energy of the individual photoelectrons increased with the frequency (or color) of the light, but was independent of the intensity (or brightness) of the radiation.
- The photoelectric current was determined by the light's intensity; doubling the intensity of the light doubled the number of emitted electrons.
This observation was very puzzling to many physicists. At the time, light was accepted as a wave phenomenon. Since energy carried by a wave should only depend on its amplitude (and not on the frequency of the wave), the frequency dependence of the emitted electrons' energies didn't make sense.
In 1905, Albert Einstein solved this apparent paradox by describing light as composed of discrete quanta (now called photons), rather than continuous waves. Building on Max Planck's theory of black body radiation, Einstein theorized that the energy in each quantum of light was equal to the frequency multiplied by a constant
According to Einstein, the maximum kinetic energy of an ejected electron is given by
Is light then composed of particles or waves? Young's experiment suggested that it was a wave, but the photoelectric effect indicated that it should be made of particles. This question would be resolved by de Broglie: light, and all matter, have both wave-like and particle-like properties.