The electric dipole moment is a measure of polarity, which is the separation of positive and negative charges in a system. It is measured in units of Coulomb-meters (C m). There are many different types of dipole moments, including electric dipole moments, magnetic dipole moments, and topological dipole moments.
Among the subset of electric dipole moments are transition dipole moments, molecular dipole moments , bond dipole moments, and electron electric dipole moments. For the purposes of this atom we will focus on a broad overview of electric dipole moment in static situations.
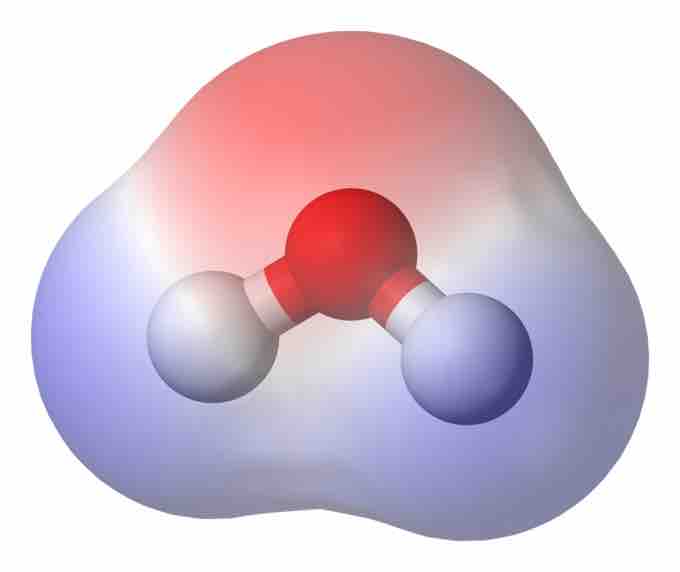
Molecular Dipole Moment in Water
This water (H2O) molecule has a high density of electrons (denoted by the red shading) near the red O atom. Closer to the white H atoms, there is a low density of electrons. Therefore, the molecule is is a dipole, with negativity near the O and positivity closer to the H atoms.
Definition
Fundamentally, for the case of point charges with values +q and -q, an electric dipole moment (p) can be defined as the vector product of the charges and the displacement vector d:
The displacement vector is the vector with a magnitude equal to the distance between the charges and a direction pointing from the negative charge to the positive charge. It is essentially interchangeable with the "radius" variable in many other equations (such as those determining gravitational and electrostatic forces), except that it includes the factor of direction.
Torque
All dipoles will experience a torsional force, or torque, when they are placed in external electric fields. This torque rotates the dipole to align it with the field. It is brought on by the need to minimize potential energy. Torque (τ) can be calculated as the cross product of the electric dipole moment and the electric field (E), assuming that E is spatially uniform: