Creutzfeldt–Jakob disease, or CJD, is a degenerative neurological disorder (brain disease) that is incurable and invariably fatal. CJD is occasionally called a human form of mad cow disease (bovine spongiform encephalopathy or BSE), even though classic CJD is not related to BSE. However, given that BSE is believed to be the cause of variant Creutzfeldt–Jakob disease (vCJD) in humans, the two are often confused. In CJD, the brain tissue develops holes and takes on a sponge-like texture. This is due to a type of infectious protein called a prion . Prions are misfolded proteins which replicate by converting their properly folded counterparts.
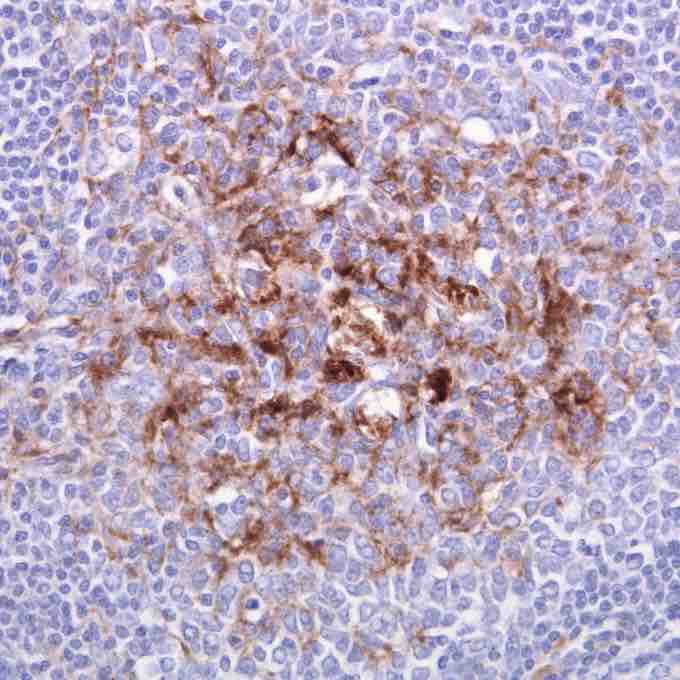
A tonsil biopsy
The brown staining is for the prion protein responsible for variant Creutzfeldt–Jakob disease prion.
Transmissible spongiform encephalopathy diseases are caused by prions. Thus, the diseases are sometimes called prion diseases. Other prion diseases include Gerstmann–Sträussler–Scheinker syndrome (GSS), fatal familial insomnia (FFI) and Kuru in humans; as well as bovine spongiform encephalopathy (BSE, commonly known as mad cow disease) in cattle, chronic wasting disease (CWD) in elk and deer, and Scrapie in sheep. Alpers' syndrome in infants is also thought to be a transmissible spongiform encephalopathy caused by a prion.
The prion that is believed to cause Creutzfeldt–Jakob exhibits at least two stable conformations. One, the native state, is water-soluble and present in healthy cells. As of 2007, its biological function is presumably in transmembrane transport or signaling. The other conformational state is relatively water-insoluble and readily forms protein aggregates. People can also acquire CJD genetically through a mutation of the gene that codes for the prion protein (PRNP). This occurs in only 5–10% of all CJD cases.
The CJD prion is dangerous because it promotes refolding of native proteins into the diseased state. The number of misfolded protein molecules will increase exponentially and the process leads to a large quantity of insoluble protein in affected cells. This mass of misfolded proteins disrupts cell function and causes cell death. Mutations in the gene for the prion protein can cause a misfolding of the dominantly alpha helical regions into beta pleated sheets. This change in conformation disables the ability of the protein to undergo digestion. Once the prion is transmitted, the defective proteins invade the brain and are produced in a self-sustaining feedback loop.