Definition of Heat
When energy is exchanged between thermodynamic systems by thermal interaction, the transfer of energy is called heat. The units of heat are therefore the units of energy, or joules (J). Heat is transferred by conduction, convection, and/or radiation.
Heat is transfer by conduction occurs when an object with high thermal energy comes into contact with an object with low thermal energy. Heat transfer by convection occurs through a medium. For example, when heat transfers from the hot water at the bottom of the pot to the cooler water at the top of the pot. Lastly, heat can also be transferred by radiation; a hot object can convey heat to anything in its surroundings via electromagnetic radiation.
When a high temperature body is brought into contact with a low temperature body, the temperatures equilibrate: there is heat flow from higher to lower temperature, like water flowing downhill, until the temperatures of the bodies are equivalent. The high temperature body loses thermal energy, and the low temperature body acquires this same amount of thermal energy. The system is then said to be at thermal equilibrium.
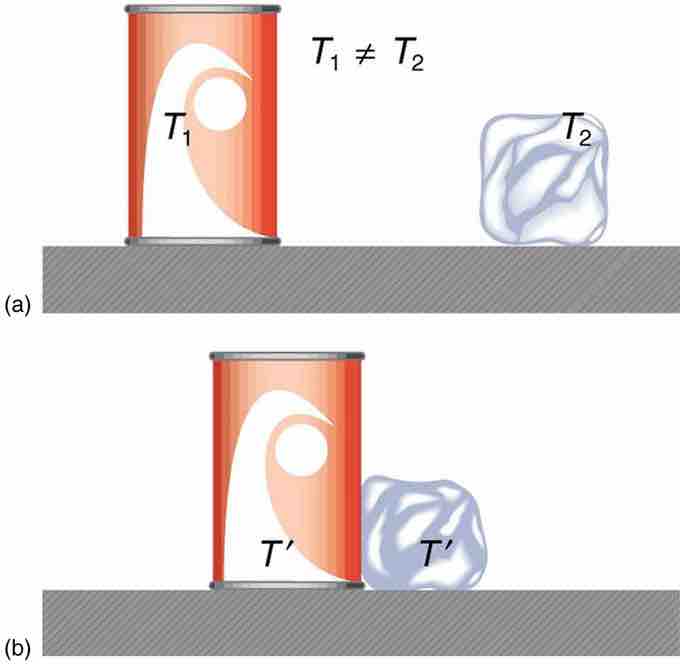
An illustration of thermal equilibrium
The can of cola and ice cube start at different temperatures. When they come into contact, heat is transferred from the cola can to the ice cube until both bodies reach thermal equilibrium.
Definition of Work
Work is the transfer of energy by any process other than heat. Like heat, the unit measurement for work is joules (J). There are many forms of work, including but not limited to mechanical, electrical, and gravitational work. For our purposes, we are concerned with P-V work, which is the work done in an enclosed chemical system. In this type of system, work is defined as the change in the volume (V) in liters within the system multiplied by a pressure (P). Assuming the system is at constant pressure, this equates to the following:
Most often, we are interested in the work done by expanding gases. Assuming the gases are ideal, we can apply the ideal gas law to the above equation to get the following:
Relationship Between Heat and Work
Heat and work are related. Work can be completely converted into heat, but the reverse is not true: heat energy cannot be wholly transformed into work energy. Scientists and engineers have been able to exploit the principles of thermochemistry to develop technologies ranging from hot/cold packs to gasoline powered combustion engines.
For a closed system, the change in internal energy (∆U) is related to heat (Q) and work (W) as follows:
This means that the total energy within a system is affected by the sum of two possible energy transfers: heat and work.