Enthalpy (H) is a measure of the energy in a system, and the change in enthalpy is denoted by
A thermochemical equation is a balanced stoichiometric chemical equation which includes the enthalpy change. The equations take the form:
Thermochemical Equations for Endothermic Reactions
The sign of the
Notice that in an endothermic reaction like the one depicted above, we can think of heat as being a reactant, just like A and B.
Thermochemical Equations for Exothermic Reactions
In an exothermic system, the
Notice that here, we can think of heat as being a product in the reaction.
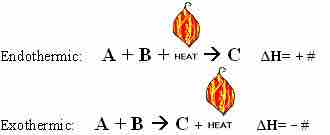
Thermochemical equations
Thermochemical equations can describe endothermic or exothermic reactions.