All chemical processes are accompanied by energy changes. When a reaction proceeds, it either releases energy to, or absorbs energy from, its surroundings. In thermodynamics, these two types of reactions are classified as exothermic or endothermic, respectively. An easy way to remember the difference between these two reaction types is by their prefixes: endo- means to draw in, and exo- means to give off. We will explore these concepts in more detail after introducing the concept of enthalpy.
Enthalpy
Enthalpy (signified as H) is a measure of the total energy of a system and often expresses and simplifies energy transfer between systems. Since the total enthalpy of a system cannot be measured directly, we most often refer to the change in enthalpy for a particular chemical reaction. At constant pressure, the change in enthalpy is equal to the heat given off, or the heat absorbed, in a given chemical reaction:
Due to this relation, the change in enthalpy,
Exothermic Reactions
Exothermic reactions are reactions or processes that release energy, usually in the form of heat or light. In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants. For this reason, the change in enthalpy,
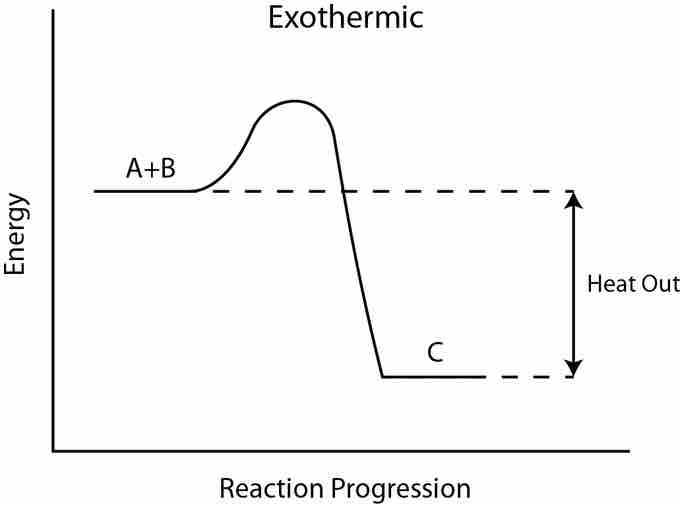
Exothermic reaction
In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings.
Endothermic Reactions
Endothermic reactions are reactions that require external energy, usually in the form of heat, for the reaction to proceed. Since endothermic reactions draw in heat from their surroundings, they tend to cause their environments to cool down. They are also generally non-spontaneous, since endothermic reactions yield products that are higher in energy than the reactants. As such, the change in enthalpy for an endothermic reaction is always positive. In order to melt the ice cube, heat is required, so the process is endothermic.
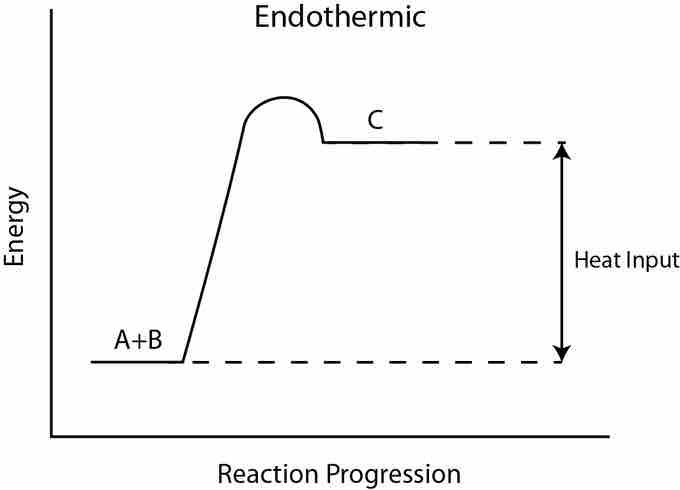
Endothermic reaction
In an endothermic reaction, the products are higher in energy than the reactants. Therefore, the change in enthalpy is positive, and heat is absorbed from the surroundings by the reaction.
Whether a reaction is endothermic or exothermic depends on the direction that it is going; some reactions are reversible, and when you revert the products back to reactants, the change in enthalpy is opposite.