The Energetics of Solution Formation
Solubility depends on dissolution of the solute into the solvent and, like all chemical reactions, is governed by the laws of thermodynamics. This particular process is a change of state from the solute's starting state, either solid, liquid or gas, to a dissolved state (termed aqueous when the solvent is water), which is a distinct physical state and thus is considered a chemical reaction. In order for any chemical reaction to proceed, it must be thermodynamically favorable. Many factors influence how thermodynamically favorable a given reaction is, including the heat of hydration, or hydration energy released when water solvates, or surrounds, an ion, and the amount of energy required to overcome the attractive forces between solute molecules, known as lattice energy.
Solvent-Solute Interactions
Since the coulombic forces that bind ions and highly polar molecules into solids are quite strong, we might expect these solids to be insoluble in most solvents. The attractive interactions between ionic molecules are called the lattice energy, and they must be overcome for a solution to form. Ionic solids are insoluble in the majority of non-aqueous solvents, but they tend to have high solubility specifically in water.
The key factor that determines solubility is the interaction of the ions with the solvent. The electrically-charged ions undergo ion-dipole interactions with water to overcome strong coulombic attraction, and this produces an aqueous solution. The water molecule is polar; it has a partial positive charge on the hydrogens while oxygen bears a partial negative charge. This dipole arises from the disparity in electronegativity present in the O-H bonds within the water molecule. Furthermore, the two lone pairs on the oxygen in water also contribute to the stabilization of any positively charged ions in solution.
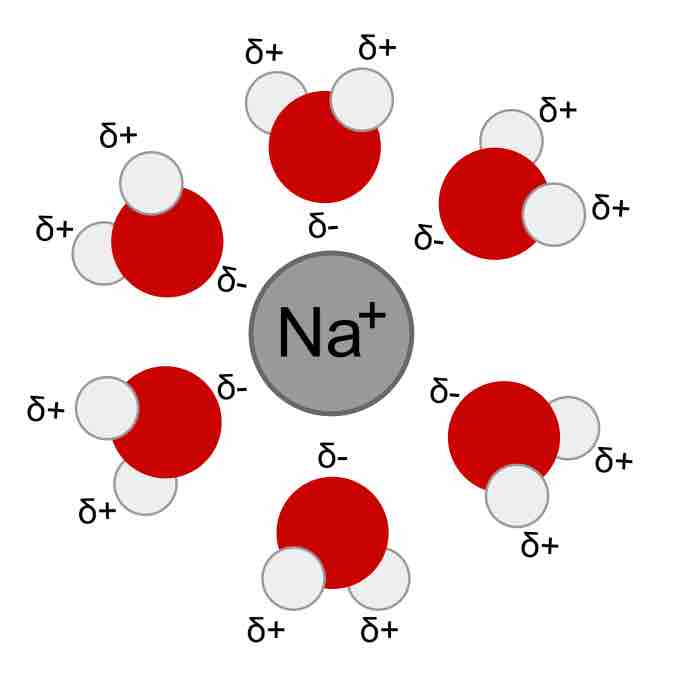
Hydrated Na+H2O cation
Water molecules surround and stabilize a cation via interaction with the partial negative charge on the oxygen ends.
As a consequence, ions in aqueous solutions are always hydrated; that is, they are quite tightly bound to water molecules through ion-dipole interactions. The number of water molecules contained in the primary hydration shell, which completely encompasses the ion, varies with the radius and charge of the ion.
Lattice Energy
The dissolution of an ionic solid MX in water can be thought of as a sequence of two processes:
The first reaction (ionization) is always endothermic; it takes a lot of work to break up an ionic crystal lattice into its component ions. Lattice energy is defined as the energy that is released when one mole of ionic solid is formed from gaseous ions, and it increases with increasing atomic charge and decreasing atomic size (radii). The greater the value of a compound's lattice energy, the greater the force required to overcome coulombic attraction. In fact, some compounds are strictly insoluble due to their high lattice energies that cannot be overcome to form a solution.
Heat of Hydration (Hhydration) vs Lattice Energy
The hydration step in the second reaction is always exothermic (Hhydration < 0) as H2O molecules are attracted into the electrostatic field of the ion. The heat (enthalpy) of solution (Hsolution) is the sum of the lattice and hydration energies ( Hsolution = Hhydration + Hlattice energy). From this relationship, we can clearly see that the processes of overcoming the lattice energy and hydrating the ions are in competition with one another.
The value of Hsolution is dependent upon the magnitudes of Hhydration and Hlattice energy of the solute. Favorable conditions for solution formation typically involve a negative value of Hsolution; this arises because the hydration process exceeds the lattice energy in the solute. As often happens for a quantity that is the sum of two large terms having opposite signs, the overall dissolution process can be either endothermic or exothermic. Hsolution is just one of the factors determining solution formation, but it is typically the major consideration in solution formation because of the role that enthalpy plays in most thermodynamic considerations.
The average time an ion spends in a hydration shell is about two to four nanoseconds, which is about two orders of magnitude longer than the lifetime of an individual H2O–H2O hydrogen bond. The relative strengths of these two intermolecular forces is apparent: ion-dipole interactions are stronger than hydrogen bond interactions.
In case you were wondering where we got the term "heat of hydration," it has to do with the fact that some solutions are highly exothermic when formed. A hot solution results when the heat of hydration is much greater than the lattice energy of the solute.
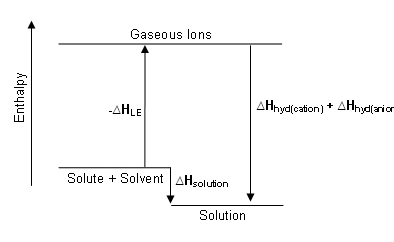
Enthalpy diagram for the dissolution process
The enthalpy diagram showing exothermic solution formation. Notice that Hsolution is lower in energy than the starting solute/solvent enthalpies. An endothermic process, on the other hand, would show Hsolution as positive, and it would be higher in energy than the starting solute/solvent enthalpies.