Relation to Aufbau Principle
Electrons will fill the lowest energy orbitals first and then move up to higher energy orbitals only after the lower energy orbitals are full. This is referred to as the Aufbau Principle, after the scientist who proposed the concept. Although the implications are clear for orbitals of different principal quantum number (n), which are clearly of different energy, the filling order is less clear for degenerate sublevels. For example, for boron through neon, the electron filling order of the 2p orbitals follows Hund's Rule.
Hund's Rule states that:
- Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
- All of the electrons in singly occupied orbitals have the same spin.
Hund's Rule Explained
According to the first rule, electrons will always occupy an empty orbital before they pair up. Electrons are negatively charged and, as a result, they repel each other. Electrons tend to minimize repulsion by occupying their own orbital, rather than sharing an orbital with another electron. Further, quantum-mechanical calculations have shown that the electrons in singly occupied orbitals are less effectively screened or shielded from the nucleus.
For the second rule, unpaired electrons in singly occupied orbitals have the same spins. If all electrons are orbiting in the same direction, they meet less often than if some of them orbit in opposite directions. In the latter case, the repulsive force increases, which separates electrons. Therefore, spins that are aligned have lower energy.
Technically speaking, the first electron in a sublevel could be either "spin-up" or "spin-down." Once the spin of the first electron in a sublevel is chosen, the spins of all of the other electrons in that sublevel depend on that first choice. To avoid confusion, scientists always draw the first electron, and any other unpaired electron, in an orbital as "spin-up."
Applying Hund's Rule
For example, take the electron configuration for carbon: 2 electrons will pair up in the 1s orbital, 2 electrons pair up in the 2s orbital, and the remaining 2 electrons will be placed into the 2p orbitals. The correct orbital diagram, obeying Hund's Rule, will note the two 2p electrons to be unpaired in two of the three available orbitals, both with "spin-up." Since electrons always occupy an empty orbital before they fill up, it would be incorrect to draw the two 2p electrons in the same orbital, leaving open orbitals unfilled.
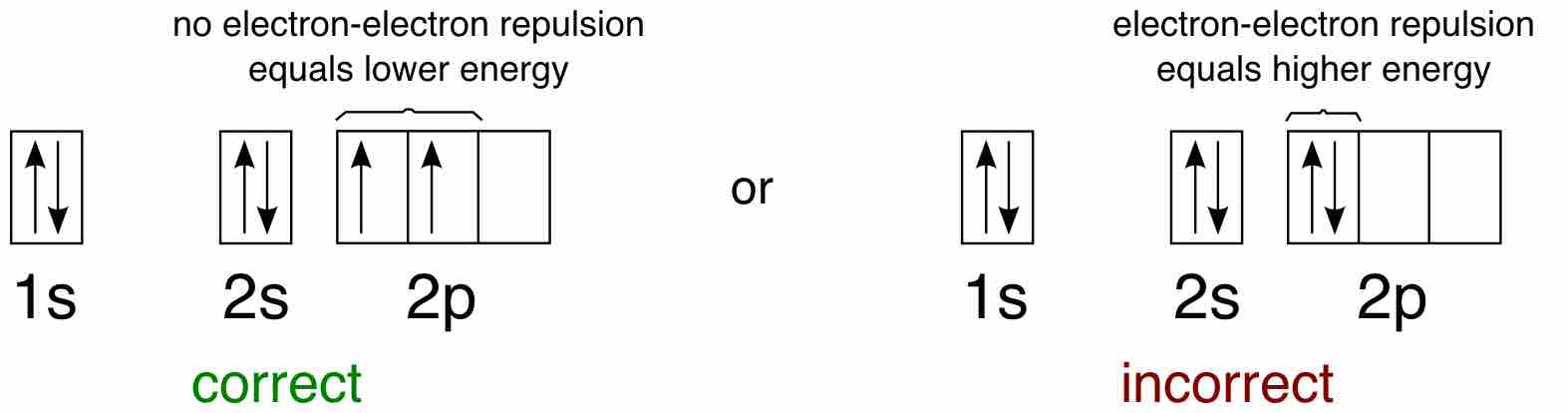
Example of Hund's rule
Orbital diagram for carbon, showing the correct application of Hund's Rule.
As another example, oxygen has 8 electrons. The electron configuration can be written as 1s22s22p4. The orbital diagram is drawn as follows: the first 2 electrons will pair up in the 1s orbital; the next 2 electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals. According to Hund's Rule, all orbitals will be singly occupied before any is doubly occupied. Therefore, two p orbitals will each get 1 electron and one will get 2 electrons. Hund's Rule also tells us that all of the unpaired electrons must have the same spin. Keeping with convention, all of the unpaired electrons are drawn as "spin-up."
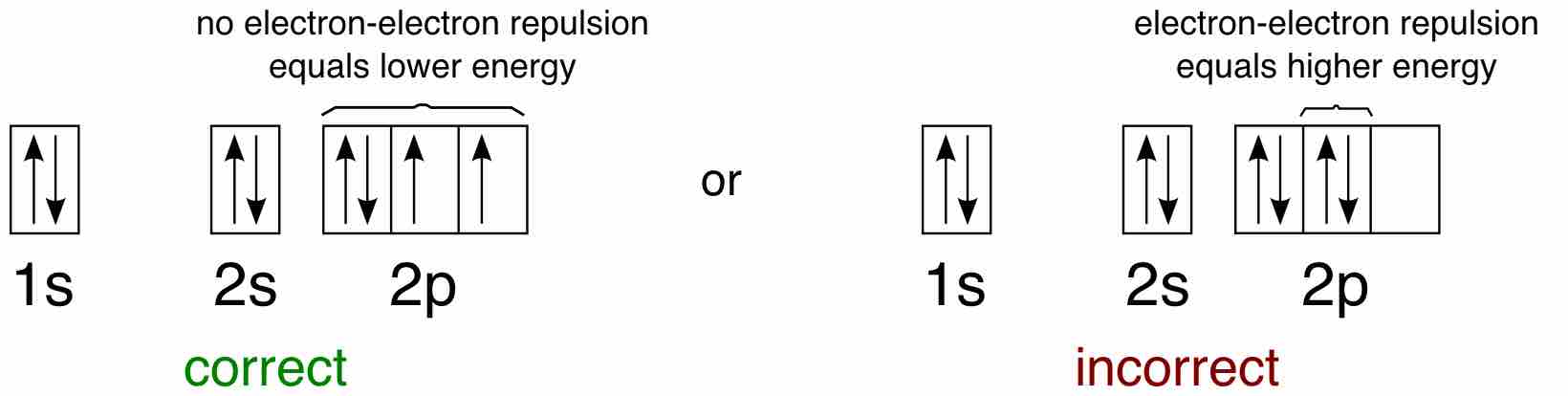
Application of Hund's rule
Orbital diagram for oxygen, which has four 2p electrons, showing the correct application of Hund's Rule.
Purpose of Electron Configurations
When atoms come into contact with one another, it is the outermost electrons of these atoms, or valence shell, that will interact first. An atom is least stable (and therefore most reactive) when its valence shell is not full. The valence electrons are largely responsible for an element's chemical behavior. Elements that have the same number of valence electrons often have similar chemical properties.
Electron configurations can also predict stability. An atom is at its most stable (and therefore unreactive) when all its orbitals are full. The most stable configurations are the ones that have full energy levels. These configurations occur in the noble gases. The noble gases are very stable elements that do not react easily with any other elements.
Electron configurations can help to make predictions about the ways in which certain elements will react and the chemical compounds or molecules that different elements will form. These principles help to understand the behavior of all chemicals, from the most basic elements like hydrogen and helium, to the most complex proteins (huge biological chemicals made of thousands of different atoms bound together) found in the human body.