In comparison to alkenes and alkynes, alkanes are relatively unreactive due to the absence of a weaker pi bond in their carbon skeletons. However, there are a few classes of reactions that are commonly performed with alkanes.
Oxidation Reactions
The most important reaction that alkanes undergo is combustion. Smaller, linear alkanes generally oxidize more readily than larger, more branched molecules. Alkanes can be burned in the presence of oxygen to produce carbon dioxide, water, and energy; in situations with limited oxygen, the products are carbon monoxide, water, and energy. For this reason, alkanes are frequently used as fuel sources. The combustion of methane is shown:
Halogenation
With the addition of a halogen gas and energy, alkanes can be halogenated with the reactivity of the halogens proceeding in the following order: Cl2>Br2>I2.
In this reaction, UV light or heat initiates a chain reaction, cleaving the covalent bond between the two atoms of a diatomic halogen. The halogen radicals can then abstract protons from the alkanes, which can then combine or react to form more radicals. Alkanes can be halogenated at a number of sites, and this reaction typically yields a mixture of halogenated products.
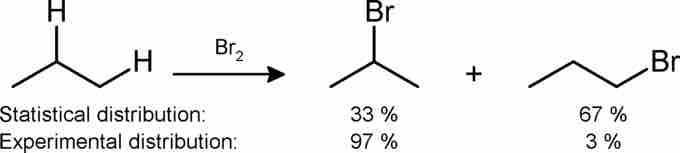
Monobromination of propane
Here, propane is brominated using diatomic bromine. The product distribution in this reaction has to do with the stability of the intermediate radicals, a topic beyond the scope of this atom.
Thermal Cracking
The complex alkanes with high molecular weights that are found in crude oil are frequently broken into smaller, more useful alkanes by thermal cracking; alkenes and hydrogen gas are also produced by using this method. Thermal cracking is typically performed at high temperatures, and often in the presence of a catalyst. A mixture of products results, and these alkanes and alkenes can be separated by fractional distillation.

Thermal cracking
A Russian factory for thermal cracking.