Hydrocarbons can be drawn in several equally valid ways. The most common method is using the bond line formula, which is ubiquitous for its simplicity.
Bond Line Structures for Hydrocarbons
To draw a hydrocarbon using the bond line method, place your pencil on a piece of paper. This first dot represents one carbon atom. To continue, draw a short, straight line. Now the hydrocarbon represented by the short, straight line is two carbon atoms in length; it has two ends.
To increase the number of carbon atoms in your drawn structure, change direction and continue with a short, straight line. In addition to the two ends, there is now a vertex that represents a third carbon atom. The angle created by the three carbon atoms should be in the region of 110 degrees.
Extending the line with more and more vertices produces a longer carbon chain.
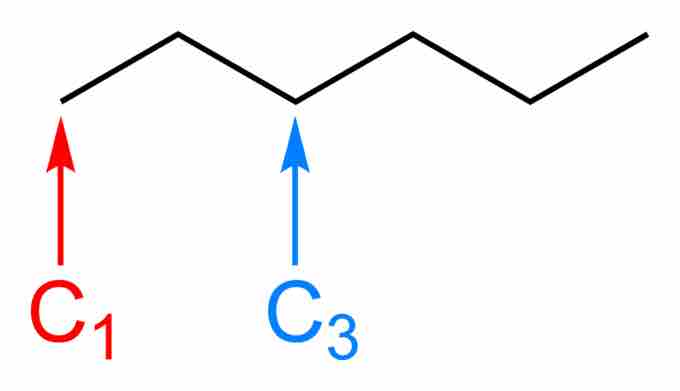
Hexane
The bond line structure of hexane can be illustrated by a kinked line with four vertices. Each vertex, as well as the two ends, represents a carbon atom.
Note that no carbon atom is explicitly written; they are implicit from the number of vertices plus the two ends. Additionally, no hydrogen atoms are written. Convention assumes that every bond line structure includes enough hydrogen atoms so that all carbon atoms in the structure make four bonds. All non-hydrogen and carbon atoms should be written explicitly, however.
Drawing Carbon Skeletons
Hydrocarbon structures are drawn starting with the carbon backbone, which is the longest branch, and are named according to the alkane series: one carbon, "meth-"; two carbons, "eth-"; three carbons, "prop-"; etc. Compounds that start with "cyclo-" are drawn starting with the ring structure.
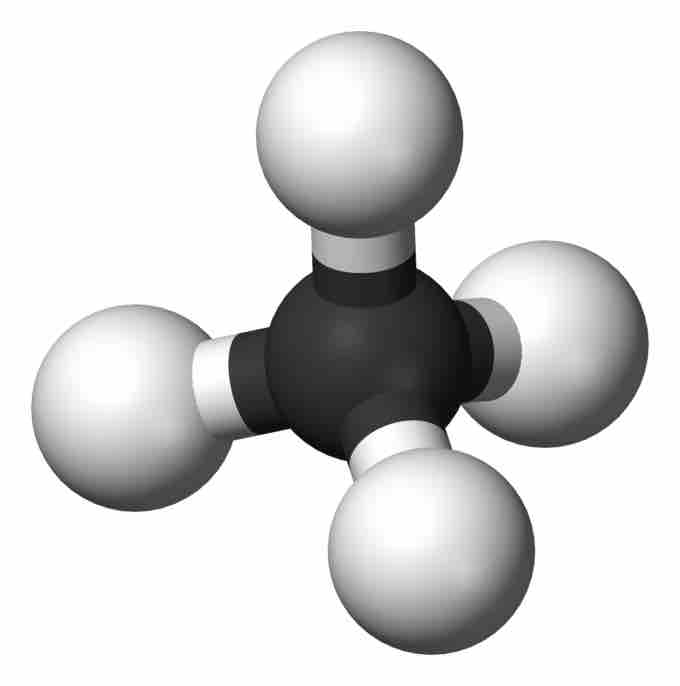
Methane
Ball-and-stick model of methane.
For example, 3-ethyl-hexane could be represented by first drawing the hexane backbone, then adding an ethyl (two-carbon) chain extending from the third carbon of the backbone.
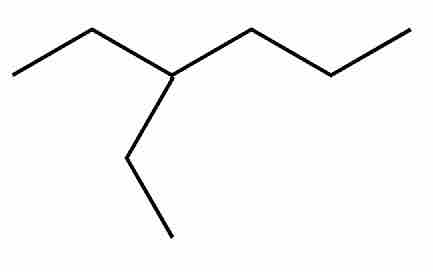
3-Ethyl hexane
Notice the two carbon chain that stems from the third carbon atom in the hexane structure.
Drawing Double and Triple Bonds
Compounds ending with "-ane" consist of solely single bonds in the carbon skeleton. The presence of double or triple bonds is determined by the suffix "-ene" or "-yne," respectively, and the positioning of the bond is defined by the numbering in the name of the structure.
When including an alkene bond in your hydrocarbon structure, aim for 120 degree bond angles about each doubly-bonded carbon. In the case of alkyne bonds, simply draw the triple bond in-line with the carbon atoms immediately bound to the alkyne carbons.

2-Pentyne
Four out of the five carbons in 2-pentyne are drawn in-line with one another.
Kekulé Structures
Kekulé structures are less commonly used than bond line structures because they are more tedious to draw. The difference is that in Kekulé structures, carbon atoms are signified with C at the vertices, and hydrogen atoms bonded to carbon are explicitly drawn.
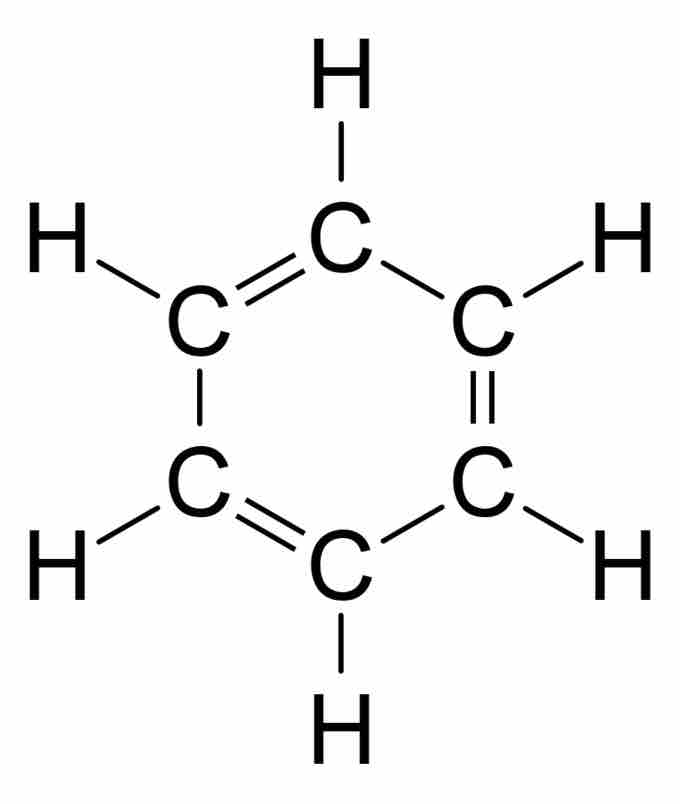
Kekulé structure for benzene
Kekuléstructure for benzene, drawn with the C for carbon atoms and with all hydrogen atoms shown.
3D Structures
The 3D configuration for substituent groups on chemical structures is often indicated by shading bonds to indicate those that are above and below the plane of the paper. These bond angles follow the sp, sp2, and sp3 hybridization patterns of the atoms in the carbon backbone.
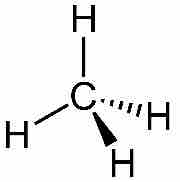
Methane structure
Notice the shading of the hydrogen bonds to indicate they are not all in the same plane.