Concept
Version 14
Created by Boundless
Real Gases
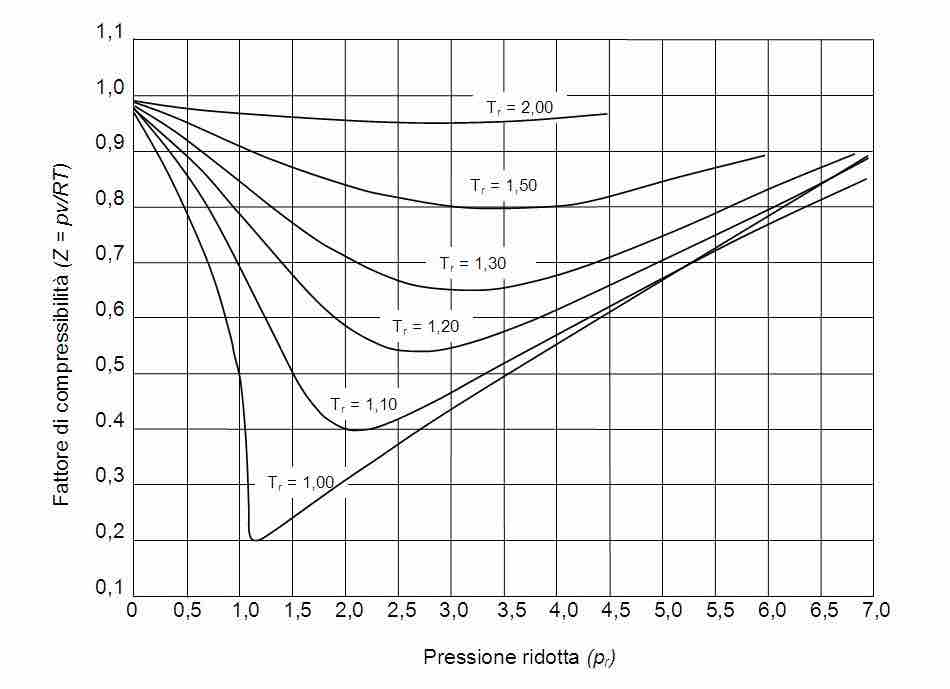
Compressibility factor and pressure
At low temperatures, the compressibility factor for a generalized gas greatly deviates from unity, indicating non-ideal gas behavior; at high temperatures, however, the compressibility factor is much less affected by the increased pressure.
Graph with vertical axis compressibility factor, horizontal axis pressure. At high temperatures, the compressibility factor is almost constant near 1 as pressure increases. At lower temperatures, the compressibility factor decreases and then increases towards 1 again as pressure increases.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Diagramma generalizzato fattore di compressibilità."
http://upload.wikimedia.org/wikipedia/commons/8/81/Diagramma_generalizzato_fattore_di_compressibilit%C3%A0.jpg
Wikipedia
CC BY-SA 3.0.