Section 7
Deviation of Gas from Ideal Behavior
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
6 concepts
The Effect of the Finite Volume
Real gases deviate from the ideal gas law due to the finite volume occupied by individual gas particles.
The Effect of Intermolecular Forces
At high pressures and low temperatures, intermolecular forces between gas particles can cause significant deviation from ideal behavior.

Van der Waals Equation
The van der Waals equation modifies the Ideal Gas Law to correct for the excluded volume of gas particles and intermolecular attractions.
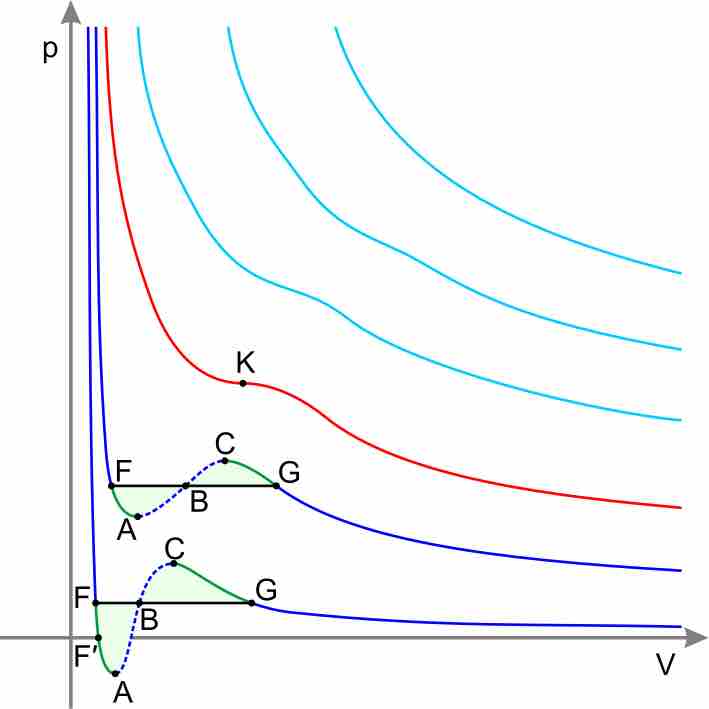
Real Gases
Equations other than the Ideal Gas Law model the non-ideal behavior of real gases at high pressures and low temperatures.

Air Pollution
Air pollution results from increasing levels of harmful molecules and particulates in the atmosphere.
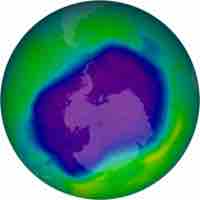
Ozone Depletion
Free radicals in the upper stratosphere act as catalysts for ozone decomposition, thereby depleting the ozone layer.