Ozone's Role in the Stratosphere
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: (1) a steady decline of about 4 percent per decade in the total volume of ozone in Earth's stratosphere (the ozone layer); and (2) a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon is referred to as the ozone hole. Both types of ozone depletion have increased as emissions of halo-carbons have increased.
The most important process in hole formation is the catalytic destruction of ozone by atomic halogens. The main source of these halogen atoms in the stratosphere is photodissociation of man-made halocarbon refrigerants; examples include CFCs (chlorofluorocarbons), freons, and halons. These compounds are emitted on Earth's surface and move into the stratosphere.
CFCs and other contributing substances are referred to as ozone-depleting substances (ODS). Since the ozone layer prevents most harmful wavelengths (280-315 nm) of ultraviolet (UV) light from passing through Earth's atmosphere, observed and projected decreases in ozone have generated worldwide concern. The Montreal Protocol bans the production of ozone-depleting chemicals such as carbon tetrachloride and trichloroethane.
Because the ozone layer acts as a protectant, blocking most UVB wavelengths of UV light from piercing Earth's atmosphere, ozone depletion may result in a variety of biological consequences; these include increased cases of skin cancer, cataracts, and plant damage, as well as reduced plankton populations in the ocean's photic zone. The largest ozone hole was observed in September, 2006.
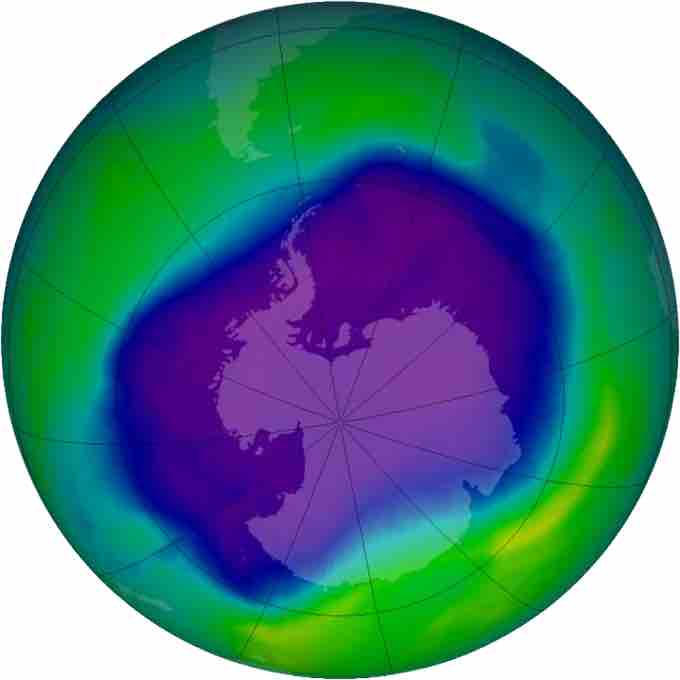
Largest ozone hole observed
In September, 2006, the average area of the ozone hole, displayed here in purple, was 10.6 million square miles.
Ozone Cycle
The ozone-oxygen cycle involves three forms of oxygen:
- Atomic oxygen (O)
- Diatomic oxygen, or oxygen gas (O2)
- Ozone (O3)
In the stratosphere, absorption of ultraviolet photons results in the photodissociation (breaking apart) of oxygen molecules. These atomic oxygen (O) radicals react with oxygen gas (O2) to produce ozone (O3); ozone's absorption of ultraviolet light can then cause oxygen gas to re-form. The balanced equation for this reaction is:
Ozone Destruction
The balance of ozone production and degradation can be disturbed by the presence of competing free radicals, most notably the hydroxyl radical (OH·), nitric oxide radical (NO·), atomic chlorine radical (Cl·), and atomic bromine radical (Br·). The reaction of these free radicals with ozone disrupts the ozone-oxygen cycle, leading to the destruction of stratospheric ozone and the depletion of the ozone layer. The atomic chlorine and bromine radicals are found in certain stable organic compounds, especially CFCs, which can make their way to the stratosphere because of their low reactivity. Once in the stratosphere, ultraviolet light liberates the Cl and Br atoms from their parent compounds:
CFCl3 + electromagnetic radiation
The Cl and Br atoms can then destroy ozone molecules through a variety of catalytic cycles. In the simplest example of such a cycle, a chlorine atom reacts with an ozone molecule, taking an oxygen atom (forming ClO, chlorine monoxide) and leaving a normal oxygen molecule (O2). The chlorine monoxide can then react with a second molecule of ozone (O3) to yield another chlorine atom and two molecules of oxygen. The chemical equations for these gas-phase reactions are:
Thus, the atomic chlorine radical regenerates; a single chlorine can keep destroying ozone (acting as a catalyst) for up to two years. The overall effect is a decrease in the amount of ozone, though null cycles can decrease the rate of these processes. More complicated mechanisms that lead to ozone destruction in the lower stratosphere have also been been discovered.
Diffusion of CFCs
A common misconception is that because CFC molecules are heavier than air (both nitrogen and oxygen), they cannot reach the stratosphere in significant amounts and therefore do not contribute significantly to ozone depletion. Atmospheric gases are not sorted by weight, however; wind forces can fully mix the gases in the atmosphere, which readily diffuse into the stratosphere.