Reduction Potential
Reduction potential (also known as redox potential, oxidation/reduction potential, or Eh) measures the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V) or millivolts (mV). Each species has its own intrinsic reduction potential. The more positive the potential, the greater the species' affinity for electrons, or the more the species tends to be reduced.
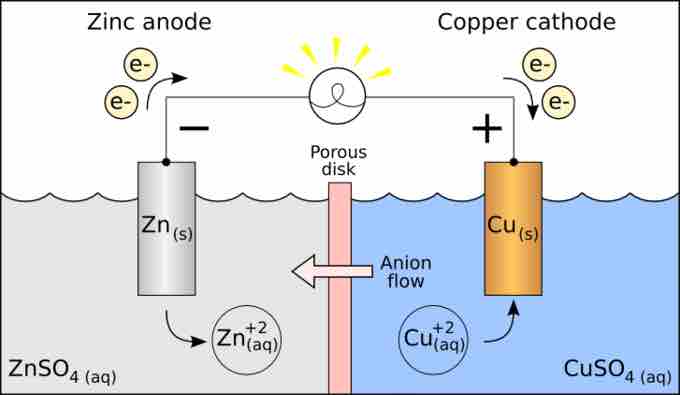
Oxidation-reduction in a galvanic cell
In this galvanic cell, zinc reduces copper cations. The reaction yields zinc cations and neutral copper metal.
The standard reduction potential (E0) is measured under standard conditions:
- 25 °C
- 1 M concentration for each ion participating in the reaction
- Partial pressure of 1 atm for each gas that is part of the reaction
- Metals in their pure states
Standard Reduction Potential
The standard reduction potential is defined relative to a standard hydrogen electrode (SHE) reference electrode, which is arbitrarily given a potential of 0.00 volts. The values below in parentheses are standard reduction potentials for half-reactions measured at 25 °C, 1 atmosphere, and with a pH of 7 in aqueous solution.
- CH3COOH + 2H+ + 2e- → CH3CHO + H2O (-0.58)
- 2H+ + 2 e- → H2 (0.0)
- O2 + 2H+ + 2e- → H2O2 (+0.7)
- O2 + 4H+ + 4e- → 2H2O (+1.64)
Since the reduction potential measures the intrinsic tendency for a species to undergo reduction, comparing standard reduction potential for two processes can be useful for determining how a reaction will proceed.
Historically, many countries, including the United States and Canada, used standard oxidation potentials rather than reduction potentials in their calculations. These are simply the negative of standard reduction potentials, so it is not a difficult conversion in practice. However, because these can also be referred to as "redox potentials," the terms "reduction potentials" and "oxidation potentials" are preferred by the IUPAC. The two may be explicitly distinguished by using the symbol E0r for reduction and E0o for oxidation.