Section 3
Standard Reduction Potentials
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
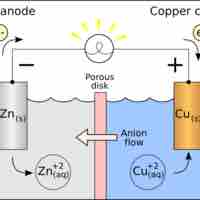
Standard Reduction Potentials
Standard reduction potentials provide a systematic measurement for different molecules' tendency to be reduced.
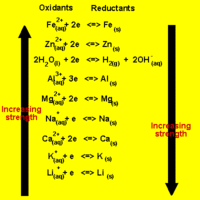
Predicting Spontaneous Direction of a Redox Reaction
The direction of a redox reaction depends on the relative strengths of the oxidants and reductants in a solution.
Predicting if a Metal Will Dissolve in Acid
A metal is soluble in acid if it displaces H2 from solution, which is determined by the metal's standard reduction potential.

Thermodynamics of Redox Reactions
The thermodynamics of redox reactions can be determined using their standard reduction potentials and the Nernst equation.