Section 4
Cell Potentials
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
The Nernst Equation
In electrochemistry, the Nernst equation can be used to determine the reduction potential of an electrochemical cell.

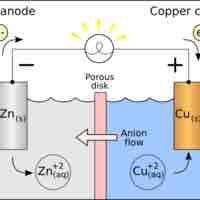
Concentration of Cells
Walther Nernst proposed a mathematical model to determine the effect of reactant concentration on the electrochemical cell potential.

Free Energy and Cell Potential
In a galvanic cell, where a spontaneous redox reaction drives the cell to produce an electric potential, the change in Gibbs free energy must be negative.
Equilibrium Constant and Cell Potential
The equilibrium constant K can be calculated using the Nernst equation.